A self-calibrating PARACEST MRI contrast agent that detects esterase enzyme activity
- PMID: 21861282
- PMCID: PMC4879975
- DOI: 10.1002/cmmi.421
A self-calibrating PARACEST MRI contrast agent that detects esterase enzyme activity
Abstract
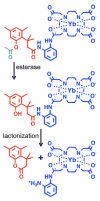
The CEST effect of many PARACEST MRI contrast agents changes in response to a molecular biomarker. However, other molecular biomarkers or environmental factors can influence CEST, so that a change in CEST is not conclusive proof for detecting the biomarker. To overcome this problem, a second control CEST effect may be included in the same PARACEST agent, which is responsive to all factors that alter the first CEST effect except for the biomarker to be measured. To investigate this approach, a PARACEST MRI contrast agent was developed with one CEST effect that is responsive to esterase enzyme activity and a second control CEST effect. The ratio of the two CEST effects was independent of concentration and T(1) relaxation, so that this agent was self-calibrating with respect to these factors. This ratiometric method was dependent on temperature and was influenced by MR coalescence as the chemical exchange rates approached the chemical shifts of the exchangable protons as temperature was increased. The two CEST effects also showed evidence of having different pH dependencies, so that this agent was not self-calibrating with respect to pH. Therefore, a self-calibrating PARACEST MRI contrast agent can more accurately detect a molecular biomarker such as esterase enzyme activity, as long as temperature and pH are within an acceptable physiological range and remain constant.
Copyright © 2010 John Wiley & Sons, Ltd.
Figures
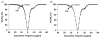
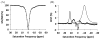
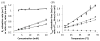
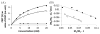
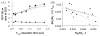
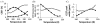
References
-
- Zhang S, Merritt M, Woesner DE, Lenkinski RE, Sherry AD. PARA-CEST agents: modulating MRI contrast via water proton exchange. Acc Chem Res. 2003;36(10):783–790. - PubMed
-
- Yoo B, Pagel MD. An overview of responsive MRI contrast agents for molecular imaging. Front Bioscience. 2008;13:1733–1752. - PubMed
-
- Ward KM, Balaban RS. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST) Magn Reson Med. 2000;44(5):799–802. - PubMed
-
- Aime S, Barge A, Delli Castelli D, Fedeli F, Mortillaro A, Nielsen FU, Terreno E. Paramagnetic lanthanide(III) complexes as pH-sensitive chemical exchange saturation transfer (CEST) contrast agents for MRI applications. Magn Reson Med. 2002;47:639–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
