Enantioselective total syntheses of (-)-palau'amine, (-)-axinellamines, and (-)-massadines
- PMID: 21861522
- PMCID: PMC3173569
- DOI: 10.1021/ja2047232
Enantioselective total syntheses of (-)-palau'amine, (-)-axinellamines, and (-)-massadines
Abstract
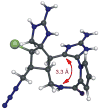
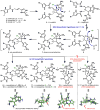
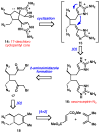
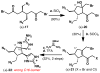
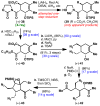
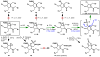
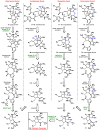
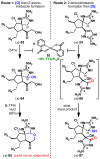
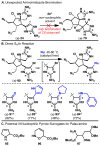
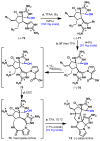
Dimeric pyrrole-imidazole alkaloids represent a rich and topologically unique class of marine natural products. This full account will follow the progression of efforts that culminated in the enantioselective total syntheses of the most structurally ornate members of this family: the axinellamines, the massadines, and palau'amine. A bio-inspired approach capitalizing on the pseudo-symmetry of the members of this class is recounted, delivering a deschloro derivative of the natural product core. Next, the enantioselective synthesis of the chlorocyclopentane core featuring a scalable, catalytic, enantioselective Diels-Alder reaction of a 1-siloxydiene is outlined in detail. Finally, the successful divergent conversion of this core to each of the aforementioned natural products, and the ensuing methodological developments, are described.
Figures
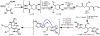






References
-
- Walker RP, Faulkner DJ, Van ED, Clardy J. J Am Chem Soc. 1981;103:6772–6773.
-
- Keifer PA, Koker MES, Schwartz RE, Hughes RG, Jr, Rittschof D, Rinehart KL. 26th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1986.
- Rinehart KL. Pure Appl Chem. 1989;61:525–528.
- Kobayashi J, Tsuda M, Murayama T, Nakamura H, Ohizumi Y, Ishibashi M, Iwamura M, Ohta T, Nozoe S. Tetrahedron. 1990;46:5579–5586.
- Keifer PA, Schwartz RE, Koker MES, Hughes RG, Jr, Rittschof D, Rinehart KL. J Org Chem. 1991;56:2965–2975.
- Williams DH, Faulkner DJ. Tetrahedron. 1996;52:5381–5390.
-
- Kinnel RB, Gehrken HP, Scheuer PJ. J Am Chem Soc. 1993;115:3376–3377.
- Kinnel RB, Gehrken HP, Swali R, Skoropowski G, Scheuer PJ. J Org Chem. 1998;63:3281–3286.
-
- Kato T, Shizuri Y, Izumida H, Yokoyama A, Endo M. Tetrahedron Lett. 1995;36:2133–2136.
- Kobayashi Ji, Suzuki M, Tsuda M. Tetrahedron. 1997;53:15681–15684.
-
-
Urban S, Leone PdA, Carroll AR, Fechner GA, Smith J, Hooper JNA, Quinn RJ. J Org Chem. 1999;64:731–735.These “axinellamines” should not be confused with the structurally unrelated natural products that share their name: Bascombe KC, Peter SR, Tinto WF, Bissada SM, McLean S, Reynolds WF. Heterocycles. 1998;48:1461–1464.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

