Extraordinarily robust polyproline type I peptoid helices generated via the incorporation of α-chiral aromatic N-1-naphthylethyl side chains
- PMID: 21861531
- PMCID: PMC3186054
- DOI: 10.1021/ja204755p
Extraordinarily robust polyproline type I peptoid helices generated via the incorporation of α-chiral aromatic N-1-naphthylethyl side chains
Abstract
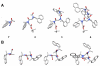
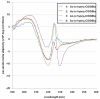
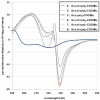
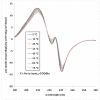
Peptoids, or oligomers of N-substituted glycines, are a class of foldamers that have shown extraordinary functional potential since their inception nearly two decades ago. However, the generation of well-defined peptoid secondary structures remains a difficult task. This challenge is due, in part, to the lack of a thorough understanding of peptoid sequence-structure relationships and, consequently, an incomplete understanding of the peptoid folding process. We seek to delineate sequence-structure relationships through the systematic study of noncovalent interactions in peptoids and the design of novel amide side chains capable of such interactions. Herein, we report the synthesis and detailed structural analysis of a series of (S)-N-(1-naphthylethyl)glycine (Ns1npe) peptoid homo-oligomers by X-ray crystallography, NMR spectroscopy, and circular dichroism (CD) spectroscopy. Four of these peptoids were found to adopt well-defined structures in the solid state, with dihedral angles similar to those observed in polyproline type I (PPI) peptide helices and in peptoids with α-chiral side chains. The X-ray crystal structure of a representative Ns1npe tetramer revealed an all cis-amide helix, with approximately three residues per turn, and a helical pitch of approximately 6.0 Å. 2D-NMR analysis of the length-dependent Ns1npe series showed that these peptoids have very high overall backbone amide K(cis/trans) values in acetonitrile, indicative of conformationally homogeneous structures in solution. Additionally, CD spectroscopy studies of the Ns1npe homo-oligomers in acetonitrile and methanol revealed a striking length-dependent increase in ellipticity per amide. These Ns1npe helices represent the most robust peptoid helices to be reported, and the incorporation of (S)-N-(1-naphthylethyl)glycines provides a new approach for the generation of stable helical structure in this important class of foldamers.
Figures



References
-
- Gellman SH. Acc. Chem. Res. 1998;31:173–180.
-
- Yoo B, Kirshenbaum K. Curr. Opin. Chem. Biol. 2008;12:714–721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

