A model of early human embryonic stem cell differentiation reveals inter- and intracellular changes on transition to squamous epithelium
- PMID: 21861759
- PMCID: PMC3353739
- DOI: 10.1089/scd.2010.0578
A model of early human embryonic stem cell differentiation reveals inter- and intracellular changes on transition to squamous epithelium
Abstract
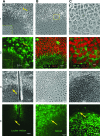
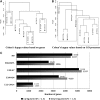
The molecular events leading to human embryonic stem cell (hESC) differentiation are the subject of considerable scrutiny. Here, we characterize an in vitro model that permits analysis of the earliest steps in the transition of hESC colonies to squamous epithelium on basic fibroblast growth factor withdrawal. A set of markers (GSC, CK18, Gata4, Eomes, and Sox17) point to a mesendodermal nature of the epithelial cells with subsequent commitment to definitive endoderm (Sox17, Cdx2, nestin, and Islet1). We assayed alterations in the transcriptome in parallel with the distribution of immunohistochemical markers. Our results indicate that the alterations of tight junctions in pluripotent culture precede the beginning of differentiation. We defined this cell population as "specified," as it is committed toward differentiation. The transitional zone between "specified" pluripotent and differentiated cells displays significant up-regulation of keratin-18 (CK18) along with a decrease in the functional activity of gap junctions and the down-regulation of 2 gap junction proteins, connexin 43 (Cx43) and connexin 45 (Cx45), which is coincidental with substantial elevation of intracellular Ca2+ levels. These findings reveal a set of cellular changes that may represent the earliest markers of in vitro hESC transition to an epithelial phenotype, before the induction of gene expression networks that guide hESC differentiation. Moreover, we hypothesize that these events may be common during the primary steps of hESC commitment to functionally varied epithelial tissue derivatives of different embryological origins.
Figures



References
-
- Murry C. Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. - PubMed
-
- Vallier L. Alexander M. Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

