Differential innate immune responses to low or high dose oral SIV challenge in Rhesus macaques
- PMID: 21861823
- PMCID: PMC3337698
- DOI: 10.2174/157016211797635928
Differential innate immune responses to low or high dose oral SIV challenge in Rhesus macaques
Abstract
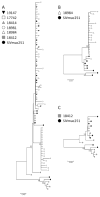
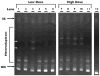
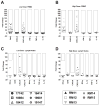
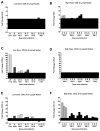
Mucosal transmission of HIV predominately occurs during sexual intercourse or breast-feeding and generally results in a successful infection from just one or few founder virions. Here we assessed the impact of viral inoculum size on both viral and immune events within two groups of Rhesus macaques that were non-traumatically, orally inoculated with either multiple low (1000 to 4000 TCID(50)) or high (100,000 TCID(50)) doses of SIV. In agreement with previous studies, more diverse SIV variants were observed in macaques following infection with high dose oral SIV compared to a low dose challenge. In peripheral blood cells, the immune gene transcript levels of CXCL9, IFNγ, TNFα and IL10 remained similar to uninfected macaques. In contrast, OAS and CXCL10 were upregulated following SIV infection in both the high and low dosed macaques, with a more rapid kinetics (detectable by 7 days) following the high SIV dose challenge. In peripheral lymph nodes, an increase in CXCL10 was observed irrespective of viral dose while CXCL9 and OAS were differentially regulated in the two SIV dosed groups. Magnetic bead sorting of CD3+, CD14+ and CD3- /CD14- cells from peripheral blood identified the increase in OAS expression primarily within CD14+ monocytes, whereas the CXCL10 expression was primarily in CD3+ T cells. These findings provide insights into the impact of SIV challenge dose on viral and innate immune factors, which has the potential to inform future SIV/HIV vaccine efficacy trials in which vaccinated hosts have the potential to be infected with a range of viral challenge doses.
Figures
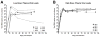
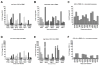

Similar articles
-
Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys.J Virol. 2009 Dec;83(23):12229-40. doi: 10.1128/JVI.01311-09. Epub 2009 Sep 16. J Virol. 2009. PMID: 19759147 Free PMC article.
-
Critical Role for Monocytes/Macrophages in Rapid Progression to AIDS in Pediatric Simian Immunodeficiency Virus-Infected Rhesus Macaques.J Virol. 2017 Aug 10;91(17):e00379-17. doi: 10.1128/JVI.00379-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28566378 Free PMC article.
-
Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251.AIDS. 1998 Jan 1;12(1):1-10. doi: 10.1097/00002030-199801000-00001. AIDS. 1998. PMID: 9456249 Free PMC article.
-
Elite Control, Gut CD4 T Cell Sparing, and Enhanced Mucosal T Cell Responses in Macaca nemestrina Infected by a Simian Immunodeficiency Virus Lacking a gp41 Trafficking Motif.J Virol. 2015 Oct;89(20):10156-75. doi: 10.1128/JVI.01134-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223646 Free PMC article.
-
Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239.J Virol. 1997 Mar;71(3):1911-21. doi: 10.1128/JVI.71.3.1911-1921.1997. J Virol. 1997. PMID: 9032322 Free PMC article.
Cited by
-
HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways.AIDS. 2013 Oct 23;27(16):2505-17. doi: 10.1097/01.aids.0000432455.06476.bc. AIDS. 2013. PMID: 24096630 Free PMC article.
-
Long-term control of simian immunodeficiency virus (SIV) in cynomolgus macaques not associated with efficient SIV-specific CD8+ T-cell responses.J Virol. 2015 Apr;89(7):3542-56. doi: 10.1128/JVI.03723-14. Epub 2015 Jan 14. J Virol. 2015. PMID: 25589645 Free PMC article.
-
Intradermal-delivered DNA vaccine induces durable immunity mediating a reduction in viral load in a rhesus macaque SARS-CoV-2 challenge model.Cell Rep Med. 2021 Oct 19;2(10):100420. doi: 10.1016/j.xcrm.2021.100420. Epub 2021 Sep 28. Cell Rep Med. 2021. PMID: 34604818 Free PMC article.
-
Functional perturbation of classical natural killer and innate lymphoid cells in the oral mucosa during SIV infection.Front Immunol. 2013 Jan 8;3:417. doi: 10.3389/fimmu.2012.00417. eCollection 2012. Front Immunol. 2013. PMID: 23316201 Free PMC article.
-
HIV-associated chronic immune activation.Immunol Rev. 2013 Jul;254(1):78-101. doi: 10.1111/imr.12079. Immunol Rev. 2013. PMID: 23772616 Free PMC article. Review.
References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. - PubMed
-
- Benmira S, Bhattacharya V, Schmid ML. An Effective HIV Vaccine: A Combination of Humoral and Cellular Immunity? Curr HIV Res. 2010;8:441–9. - PubMed
-
- Jotwani R, Cutler CW. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ Tcells in situ. J Dent Res. 2003;82:736–41. - PubMed
-
- Chou LL, Epstein J, Cassol SA, West DM, He W, Firth JD. Oral mucosal Langerhans’ cells as target, effector and vector in HIV infection. J Oral Pathol Med. 2000;29:394–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

