Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis
- PMID: 21861862
- PMCID: PMC3239343
- DOI: 10.1186/ar3380
Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis
Abstract
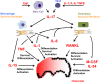
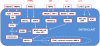
Bone destruction is a frequent and clinically serious event in patients with rheumatoid arthritis (RA). Local joint destruction can cause joint instability and often necessitates reconstructive or replacement surgery. Moreover, inflammation-induced systemic bone loss is associated with an increased fracture risk. Bone resorption is a well-controlled process that is dependent on the differentiation of monocytes to bone-resorbing osteoclasts. Infiltrating as well as resident synovial cells, such as T cells, monocytes and synovial fibroblasts, have been identified as sources of osteoclast differentiation signals in RA patients. Pro-inflammatory cytokines are amongst the most important mechanisms driving this process. In particular, macrophage colony-stimulating factor, RANKL, TNF, IL-1 and IL-17 may play dominant roles in the pathogenesis of arthritis-associated bone loss. These cytokines activate different intracellular pathways to initiate osteoclast differentiation. Thus, over the past years several promising targets for the treatment of arthritic bone destruction have been defined.
Figures
References
-
- Schett G, Kiechl S, Weger S, Pederiva A, Mayr A, Petrangeli M, Oberhollenzer F, Lorenzini R, Redlich K, Axmann R, Zwerina J, Willeit J. High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch Intern Med. 2006;166:2495–2501. doi: 10.1001/archinte.166.22.2495. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

