A glycine zipper motif mediates the formation of toxic β-amyloid oligomers in vitro and in vivo
- PMID: 21861874
- PMCID: PMC3178497
- DOI: 10.1186/1750-1326-6-61
A glycine zipper motif mediates the formation of toxic β-amyloid oligomers in vitro and in vivo
Erratum in
- Mol Neurodegener. 2014;9:12. Lacor, Pascale [corrected to Lacor, Pascale N]; Velasco, Pauline T [added]
Abstract
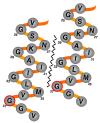
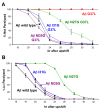
Background: The β-amyloid peptide (Aβ) contains a Gly-XXX-Gly-XXX-Gly motif in its C-terminal region that has been proposed to form a "glycine zipper" that drives the formation of toxic Aβ oligomers. We have tested this hypothesis by examining the toxicity of Aβ variants containing substitutions in this motif using a neuronal cell line, primary neurons, and a transgenic C. elegans model.
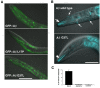
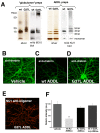
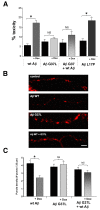
Results: We found that a Gly37Leu substitution dramatically reduced Aβ toxicity in all models tested, as measured by cell dysfunction, cell death, synaptic alteration, or tau phosphorylation. We also demonstrated in multiple models that Aβ Gly37Leu is actually anti-toxic, thereby supporting the hypothesis that interference with glycine zipper formation blocks assembly of toxic Aβ oligomers. To test this model rigorously, we engineered second site substitutions in Aβ predicted by the glycine zipper model to compensate for the Gly37Leu substitution and expressed these in C. elegans. We show that these second site substitutions restore in vivo Aβtoxicity, further supporting the glycine zipper model.
Conclusions: Our structure/function studies support the view that the glycine zipper motif present in the C-terminal portion of Aβ plays an important role in the formation of toxic Aβ oligomers. Compounds designed to interfere specifically with formation of the glycine zipper could have therapeutic potential.
Figures




Similar articles
-
Role of amyloid-beta glycine 33 in oligomerization, toxicity, and neuronal plasticity.J Neurosci. 2009 Jun 10;29(23):7582-90. doi: 10.1523/JNEUROSCI.1336-09.2009. J Neurosci. 2009. PMID: 19515926 Free PMC article.
-
In vivo induction of membrane damage by β-amyloid peptide oligomers.Acta Neuropathol Commun. 2018 Nov 29;6(1):131. doi: 10.1186/s40478-018-0634-x. Acta Neuropathol Commun. 2018. PMID: 30497524 Free PMC article.
-
Humanin Specifically Interacts with Amyloid-β Oligomers and Counteracts Their in vivo Toxicity.J Alzheimers Dis. 2017;57(3):857-871. doi: 10.3233/JAD-160951. J Alzheimers Dis. 2017. PMID: 28282805
-
[Involvement of beta-amyloid in the etiology of Alzheimer's disease].Brain Nerve. 2010 Jul;62(7):691-9. Brain Nerve. 2010. PMID: 20675873 Review. Japanese.
-
Modulation of Amyloid β-Protein (Aβ) Assembly by Homologous C-Terminal Fragments as a Strategy for Inhibiting Aβ Toxicity.ACS Chem Neurosci. 2016 Jul 20;7(7):845-56. doi: 10.1021/acschemneuro.6b00154. Epub 2016 Jul 5. ACS Chem Neurosci. 2016. PMID: 27322435 Free PMC article. Review.
Cited by
-
Key Residues for the Formation of Aβ42 Amyloid Fibrils.ACS Omega. 2018 Jul 31;3(7):8401-8407. doi: 10.1021/acsomega.8b00887. ACS Omega. 2018. PMID: 30087945 Free PMC article.
-
Vitamin B12 impacts amyloid beta-induced proteotoxicity by regulating the methionine/S-adenosylmethionine cycle.Cell Rep. 2021 Sep 28;36(13):109753. doi: 10.1016/j.celrep.2021.109753. Cell Rep. 2021. PMID: 34592146 Free PMC article.
-
Protective role of DNJ-27/ERdj5 in Caenorhabditis elegans models of human neurodegenerative diseases.Antioxid Redox Signal. 2014 Jan 10;20(2):217-35. doi: 10.1089/ars.2012.5051. Epub 2013 Jul 3. Antioxid Redox Signal. 2014. PMID: 23641861 Free PMC article.
-
Amyloid beta acts synergistically as a pro-inflammatory cytokine.Neurobiol Dis. 2021 Nov;159:105493. doi: 10.1016/j.nbd.2021.105493. Epub 2021 Aug 28. Neurobiol Dis. 2021. PMID: 34464705 Free PMC article.
-
Azepine-Indole Alkaloids From Psychotria nemorosa Modulate 5-HT2A Receptors and Prevent in vivo Protein Toxicity in Transgenic Caenorhabditis elegans.Front Neurosci. 2022 Mar 14;16:826289. doi: 10.3389/fnins.2022.826289. eCollection 2022. Front Neurosci. 2022. PMID: 35360162 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

