Characterization of upper thoracic spinal neurons receiving noxious cardiac and/or somatic inputs in diabetic rats
- PMID: 21862419
- PMCID: PMC3210409
- DOI: 10.1016/j.autneu.2011.07.007
Characterization of upper thoracic spinal neurons receiving noxious cardiac and/or somatic inputs in diabetic rats
Abstract
The aim of the present study was to examine spinal processing of cardiac and somatic nociceptive input in rats with STZ-induced diabetes. Type 1 diabetes was induced with streptozotocin (50mg/kg) in 14 male Sprague-Dawley rats and citrate buffer was injected in 14 control rats. After 4-11 weeks, the rats were anesthetized with pentobarbital, ventilated and paralyzed. A laminectomy enabled extracellular recording of T(3) spinal cord neuronal activity. Intrapericardial administration of a mixture of algogenic chemicals (bradykinin, serotonin, prostaglandin E(2) (all at 10(-5)M), and adenosine (10(-3)M)) was applied to activate nociceptors of cardiac afferent nerve endings. Furthermore, somatic receptive properties were examined by applying innocuous (brush and light pressure) and noxious (pinch) cutaneous mechanical stimuli. Diabetes-induced increases in spontaneous activity were observed in subsets of neurons exhibiting long-lasting excitatory responses to administration of the algogenic mixture. Algogenic chemicals altered activity of a larger proportion of neurons from diabetic animals (73/111) than control animals (55/115, P<0.05). Some subtypes of neurons exhibiting long-lasting excitatory responses, elicited prolonged duration and others, had a shortened latency. Some neurons exhibiting short-lasting excitatory responses in diabetic animals elicited a shorter latency and some a decreased excitatory change. The size of the somatic receptive field was increased for cardiosomatic neurons from diabetic animals. Cutaneous somatic mechanical stimulation caused spinal neurons to respond with a mixture of hyper- and hypoexcitability. In conclusion, diabetes induced changes in the spinal processing of cardiac input and these might contribute to cardiovascular autonomic neuropathy in patients with diabetes.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


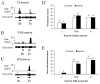

References
-
- Ahlgren SC, White DM, Levine JD. Increased responsiveness of sensory neurons in the saphenous nerve of the streptozotocin-diabetic rat. J Neurophysiol. 1992;68(6):2077–2085. - PubMed
-
- Ahluwalia G, Jain P, Chugh SK, Wasir HS, Kaul U. Silent myocardial ischemia in diabetics with normal autonomic function. Int J Cardiol. 1995;48(2):147–153. - PubMed
-
- Arendt-Nielsen L, Laursen RJ, Drewes AM. Referred pain as an indicator for neural plasticity. Prog Brain Res. 2000;129:343–356. - PubMed
-
- Biella G, Riva L, Sotgiu ML. Interaction between neurons in different laminae of the dorsal horn of the spinal cord. A correlation study in normal and neuropathic rats. Eur J Neurosci. 1997;9(5):1017–1025. - PubMed
-
- Biessels GJ, Stevens EJ, Mahmood SJ, Gispen WH, Tomlinson DR. Insulin partially reverses deficits in peripheral nerve blood flow and conduction in experimental diabetes. Journal of the Neurological Sciences. 1996;140(1–2):12–20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

