Non-redundant function of dystroglycan and β1 integrins in radial sorting of axons
- PMID: 21862561
- PMCID: PMC3160097
- DOI: 10.1242/dev.065490
Non-redundant function of dystroglycan and β1 integrins in radial sorting of axons
Abstract
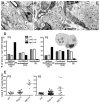
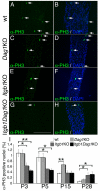
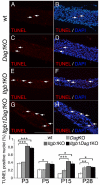
Radial sorting allows the segregation of axons by a single Schwann cell (SC) and is a prerequisite for myelination during peripheral nerve development. Radial sorting is impaired in models of human diseases, congenital muscular dystrophy (MDC) 1A, MDC1D and Fukuyama, owing to loss-of-function mutations in the genes coding for laminin α2, Large or fukutin glycosyltransferases, respectively. It is not clear which receptor(s) are activated by laminin 211, or glycosylated by Large and fukutin during sorting. Candidates are αβ1 integrins, because their absence phenocopies laminin and glycosyltransferase deficiency, but the topography of the phenotypes is different and β1 integrins are not substrates for Large and fukutin. By contrast, deletion of the Large and fukutin substrate dystroglycan does not result in radial sorting defects. Here, we show that absence of dystroglycan in a specific genetic background causes sorting defects with topography identical to that of laminin 211 mutants, and recapitulating the MDC1A, MDC1D and Fukuyama phenotypes. By epistasis studies in mice lacking one or both receptors in SCs, we show that only absence of β1 integrins impairs proliferation and survival, and arrests radial sorting at early stages, that β1 integrins and dystroglycan activate different pathways, and that the absence of both molecules is synergistic. Thus, the function of dystroglycan and β1 integrins is not redundant, but is sequential. These data identify dystroglycan as a functional laminin 211 receptor during axonal sorting and the key substrate relevant to the pathogenesis of glycosyltransferase congenital muscular dystrophies.
Figures







References
-
- Barresi R., Campbell K. P. (2006). Dystroglycan: from biosynthesis to pathogenesis of human disease. J. Cell Sci. 119, 199-207 - PubMed
-
- Benninger Y., Thurnherr T., Pereira J. A., Krause S., Wu X., Chrostek-Grashoff A., Herzog D., Nave K. A., Franklin R. J., Meijer D., et al. (2007). Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J. Cell Biol. 177, 1051-1061 - PMC - PubMed
-
- Bhat N. R., Zhang P., Mohanty S. B. (2007). p38 MAP kinase regulation of oligodendrocyte differentiation with CREB as a potential target. Neurochem.Res. 32, 293-302 - PubMed
-
- Bradley W. G., Jenkison M. (1973). Abnormalities of peripheral nerves in murine muscular dystrophy. J. Neurol. Sci. 18, 227-247 - PubMed
-
- Bradley W. G., Jenkison M. (1975). Neural abnormalities in the dystrophic mouse. J. Neurol. Sci. 25, 249-255 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

