Caenorhabditis elegans UCP4 protein controls complex II-mediated oxidative phosphorylation through succinate transport
- PMID: 21862587
- PMCID: PMC3199514
- DOI: 10.1074/jbc.M111.271452
Caenorhabditis elegans UCP4 protein controls complex II-mediated oxidative phosphorylation through succinate transport
Abstract
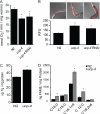
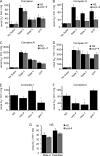
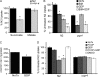
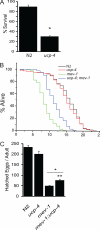
The novel uncoupling proteins (UCP2-5) are implicated in the mitochondrial control of oxidant production, insulin signaling, and aging. Attempts to understand their functions have been complicated by overlapping expression patterns in most organisms. Caenorhabditis elegans nematodes are unique because they express only one UCP ortholog, ceUCP4 (ucp4). Here, we performed detailed metabolic analyzes in genetically modified nematodes to define the function of the ceUCP4. The knock-out mutant ucp4 (ok195) exhibited sharply decreased mitochondrial succinate-driven (complex II) respiration. However, respiratory coupling and electron transport chain function were normal in ucp4 mitochondria. Surprisingly, isolated ucp4 mitochondria showed markedly decreased succinate uptake. Similarly, ceUCP4 inhibition blocked succinate respiration and import in wild type mitochondria. Genetic and pharmacologic inhibition of complex I function was selectively lethal to ucp4 worms, arguing that ceUCP4-regulated succinate transport is required for optimal complex II function in vivo. Additionally, ceUCP4 deficiency prolonged lifespan in the short-lived mev1 mutant that exhibits complex II-generated oxidant production. These results identify a novel function for ceUCP4 in the regulation of complex II-based metabolism through an unexpected mechanism involving succinate transport.
Figures
Similar articles
-
Uncoupling protein-4 (UCP4) increases ATP supply by interacting with mitochondrial Complex II in neuroblastoma cells.PLoS One. 2012;7(2):e32810. doi: 10.1371/journal.pone.0032810. Epub 2012 Feb 29. PLoS One. 2012. PMID: 22427795 Free PMC article.
-
Identification of a previously undetected metabolic defect in the Complex II Caenorhabditis elegans mev-1 mutant strain using respiratory control analysis.Biogerontology. 2017 Apr;18(2):189-200. doi: 10.1007/s10522-016-9672-6. Epub 2016 Dec 30. Biogerontology. 2017. PMID: 28039571
-
Examination of the requirement for ucp-4, a putative homolog of mammalian uncoupling proteins, for stress tolerance and longevity in C. elegans.Mech Ageing Dev. 2005 Oct;126(10):1090-6. doi: 10.1016/j.mad.2005.04.002. Mech Ageing Dev. 2005. PMID: 15893362 Free PMC article.
-
Mitochondrial respiration and reactive oxygen species in C. elegans.Exp Gerontol. 2006 Oct;41(10):957-67. doi: 10.1016/j.exger.2006.06.056. Epub 2006 Aug 21. Exp Gerontol. 2006. PMID: 16919906 Review.
-
Effects of the mitochondrial respiratory chain on longevity in C. elegans.Exp Gerontol. 2014 Aug;56:245-55. doi: 10.1016/j.exger.2014.03.028. Epub 2014 Apr 5. Exp Gerontol. 2014. PMID: 24709342 Review.
Cited by
-
Perspectives on mitochondrial uncoupling proteins-mediated neuroprotection.J Bioenerg Biomembr. 2015 Apr;47(1-2):119-31. doi: 10.1007/s10863-014-9580-x. Epub 2014 Sep 14. J Bioenerg Biomembr. 2015. PMID: 25217852 Review.
-
The addition of a developmental factor, unc-62, to already long-lived worms increases lifespan and healthspan.Biol Open. 2017 Dec 15;6(12):1796-1801. doi: 10.1242/bio.027433. Biol Open. 2017. PMID: 29055022 Free PMC article.
-
Comparison of proteomic and metabolomic profiles of mutants of the mitochondrial respiratory chain in Caenorhabditis elegans.Mitochondrion. 2015 Jan;20:95-102. doi: 10.1016/j.mito.2014.12.004. Epub 2014 Dec 19. Mitochondrion. 2015. PMID: 25530493 Free PMC article.
-
Thriving in Oxygen While Preventing ROS Overproduction: No Two Systems Are Created Equal.Front Physiol. 2022 Apr 4;13:874321. doi: 10.3389/fphys.2022.874321. eCollection 2022. Front Physiol. 2022. PMID: 35444563 Free PMC article. Review.
-
Drosophila melanogaster Uncoupling Protein-4A (UCP4A) Catalyzes a Unidirectional Transport of Aspartate.Int J Mol Sci. 2022 Jan 18;23(3):1020. doi: 10.3390/ijms23031020. Int J Mol Sci. 2022. PMID: 35162943 Free PMC article.
References
-
- Cannon B., Hedin A., Nedergaard J. (1982) FEBS Lett. 150, 129–132 - PubMed
-
- Krauss S., Zhang C. Y., Lowell B. B. (2005) Nat. Rev. Mol. Cell Biol. 6, 248–261 - PubMed
-
- Heaton G. M., Wagenvoord R. J., Kemp A., Jr., Nicholls D. G. (1978) Eur. J. Biochem. 82, 515–521 - PubMed
-
- Enerback S., Jacobsson A., Simpson E. M., Guerra C., Yamashita H., Harper M. E., Kozak L. P. (1997) Nature 387, 90–94 - PubMed
-
- Hanák P., Jezek P. (2001) FEBS Lett. 495, 137–141 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

