Histone 3 lysine 9 (H3K9) methyltransferase recruitment to the interleukin-2 (IL-2) promoter is a mechanism of suppression of IL-2 transcription by the transforming growth factor-β-Smad pathway
- PMID: 21862595
- PMCID: PMC3195630
- DOI: 10.1074/jbc.M111.236794
Histone 3 lysine 9 (H3K9) methyltransferase recruitment to the interleukin-2 (IL-2) promoter is a mechanism of suppression of IL-2 transcription by the transforming growth factor-β-Smad pathway
Abstract
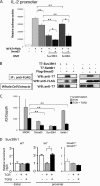
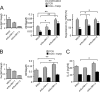
Suppression of IL-2 βproduction from T cells is an important process for the immune regulation by TGF-β. However, the mechanism by which this suppression occurs remains to be established. Here, we demonstrate that Smad2 and Smad3, two major TGF-β-downstream transcription factors, are redundantly essential for TGF-β-mediated suppression of IL-2 production in CD4(+) T cells using Smad2- and Smad3-deficient T cells. Both Smad2 and Smad3 were recruited into the proximal region of the IL-2 promoter in response to TGF-β. We then investigated the histone methylation status of the IL-2 promoter. Although both histone H3 lysine 9 (H3K9) and H3K27 trimethylation have been implicated in gene silencing, only H3K9 trimethylation was increased in the proximal region of the IL-2 promoter in a Smad2/3-dependent manner, whereas H3K27 trimethylation was not. The H3K9 methyltransferases Setdb1 and Suv39h1 bound to Smad3 and suppressed IL-2 promoter activity in collaboration with Smad3. Overexpression of Suv39h1 in 68-41 T cells strongly inhibited IL-2 production in response to T cell receptor stimulation irrespective of the presence or absence of TGF-β, whereas Setdb1 overexpression only slightly suppressed IL-2 production. Silencing of Suv39h1 by shRNA reverted the suppressive effect of TGF-β on IL-2 production. Furthermore, TGF-β induced Suv39h1 recruitment to the proximal region of the IL-2 promoter in wild type primary T cells; however, this was not observed in Smad2(-/-)Smad3(+/-) T cells. Thus, we propose that Smads recruit H3K9 methyltransferases Suv39h1 to the IL-2 promoter, thereby inducing suppressive histone methylation and inhibiting T cell receptor-mediated IL-2 transcription.
Figures




Similar articles
-
Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development.J Immunol. 2010 Jul 15;185(2):842-55. doi: 10.4049/jimmunol.0904100. Epub 2010 Jun 14. J Immunol. 2010. PMID: 20548029
-
Silencing of the Il2 gene transcription is regulated by epigenetic changes in anergic T cells.Eur J Immunol. 2012 Sep;42(9):2471-83. doi: 10.1002/eji.201142307. Epub 2012 Jul 13. Eur J Immunol. 2012. PMID: 22684523 Free PMC article.
-
ThPOK represses CXXC5, which induces methylation of histone H3 lysine 9 in Cd40lg promoter by association with SUV39H1: implications in repression of CD40L expression in CD8+ cytotoxic T cells.J Leukoc Biol. 2016 Aug;100(2):327-38. doi: 10.1189/jlb.1A0915-396RR. Epub 2016 Feb 19. J Leukoc Biol. 2016. PMID: 26896487
-
A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling.J Cell Biochem. 2007 May 1;101(1):9-33. doi: 10.1002/jcb.21255. J Cell Biochem. 2007. PMID: 17340614 Review.
-
Receptor-activated transcription factors and beyond: multiple modes of Smad2/3-dependent transmission of TGF-β signaling.J Biol Chem. 2024 May;300(5):107256. doi: 10.1016/j.jbc.2024.107256. Epub 2024 Apr 2. J Biol Chem. 2024. PMID: 38569937 Free PMC article. Review.
Cited by
-
Epigenetics within the matrix: a neo-regulator of fibrotic disease.Epigenetics. 2012 Sep;7(9):987-93. doi: 10.4161/epi.21567. Epub 2012 Aug 16. Epigenetics. 2012. PMID: 22894907 Free PMC article. Review.
-
The role of histone methylase and demethylase in antitumor immunity: A new direction for immunotherapy.Front Immunol. 2023 Jan 11;13:1099892. doi: 10.3389/fimmu.2022.1099892. eCollection 2022. Front Immunol. 2023. PMID: 36713412 Free PMC article. Review.
-
DGCR14 induces Il17a gene expression through the RORγ/BAZ1B/RSKS2 complex.Mol Cell Biol. 2015 Jan;35(2):344-55. doi: 10.1128/MCB.00926-14. Epub 2014 Nov 3. Mol Cell Biol. 2015. PMID: 25368387 Free PMC article.
-
Structure, Activity and Function of the SETDB1 Protein Methyltransferase.Life (Basel). 2021 Aug 11;11(8):817. doi: 10.3390/life11080817. Life (Basel). 2021. PMID: 34440561 Free PMC article. Review.
-
Crosstalk Between Inflammatory Signaling and Methylation in Cancer.Front Cell Dev Biol. 2021 Nov 24;9:756458. doi: 10.3389/fcell.2021.756458. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901003 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

