Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring
- PMID: 21862611
- PMCID: PMC3199015
- DOI: 10.1210/en.2011-1279
Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring
Abstract
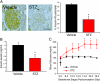
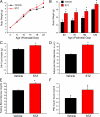
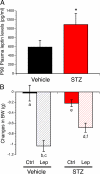
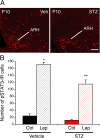
Maternal diabetes is a common complication of pregnancy, and the offspring of diabetic mothers have a higher risk of developing obesity and type 2 diabetes later in life. Despite these observations, the precise biological processes mediating this metabolic programming are not well understood. Here, we explored the consequences of maternal diabetes on the organization of hypothalamic neural circuits involved in the regulation of energy balance. To accomplish this aim, we used a mouse model of maternal insulin deficiency induced by streptozotocin injections. Maternal diabetes was found to be associated with changes in offspring growth as revealed by a significantly higher pre- and postweaning body weight in the offspring of insulin-deficient dams relative to those of control mice. Mice born to diabetic dams also showed increased fasting glucose levels, increased insulin levels, and increased food intake during their adult lives. These impairments in metabolic regulation were associated with leptin resistance during adulthood. Importantly, the ability of leptin to activate intracellular signaling in arcuate neurons was also significantly reduced in neonates born to diabetic dams. Furthermore, neural projections from the arcuate nucleus to the paraventricular nucleus were markedly reduced in the offspring of insulin-deficient dams. Together, these data show that insulin deficiency during gestation has long-term consequences for metabolic regulation. They also indicate that animals born to diabetic dams display abnormally organized hypothalamic feeding pathways that could result from the attenuated responsiveness of hypothalamic neurons to the neurotrophic actions of leptin during neonatal development.
Figures


Comment in
-
Sugaring appetite development: mechanisms of neuroendocrine programming.Endocrinology. 2011 Nov;152(11):4007-9. doi: 10.1210/en.2011-1659. Endocrinology. 2011. PMID: 22021197 No abstract available.
Similar articles
-
Sugaring appetite development: mechanisms of neuroendocrine programming.Endocrinology. 2011 Nov;152(11):4007-9. doi: 10.1210/en.2011-1659. Endocrinology. 2011. PMID: 22021197 No abstract available.
-
Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways.Mol Metab. 2020 Dec;42:101079. doi: 10.1016/j.molmet.2020.101079. Epub 2020 Sep 9. Mol Metab. 2020. PMID: 32919096 Free PMC article.
-
Differential hypothalamic leptin sensitivity in obese rat offspring exposed to maternal and postnatal intake of chocolate and soft drink.Nutr Diabetes. 2017 Jan 16;7(1):e242. doi: 10.1038/nutd.2016.53. Nutr Diabetes. 2017. PMID: 28092346 Free PMC article.
-
Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities.Front Neuroendocrinol. 2003 Jan;24(1):1-10. doi: 10.1016/s0091-3022(02)00105-x. Front Neuroendocrinol. 2003. PMID: 12609497 Review.
-
Early life programming of obesity: the impact of the perinatal environment on the development of obesity and metabolic dysfunction in the offspring.Curr Diabetes Rev. 2012 Jan;8(1):55-68. doi: 10.2174/157339912798829214. Curr Diabetes Rev. 2012. PMID: 22352445 Review.
Cited by
-
Early life overnutrition impairs plasticity of non-neuronal brainstem cells and drives obesity in offspring across development in rats.Int J Obes (Lond). 2020 Dec;44(12):2405-2418. doi: 10.1038/s41366-020-00658-5. Epub 2020 Sep 30. Int J Obes (Lond). 2020. PMID: 32999409
-
Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes.J Clin Invest. 2012 Nov;122(11):3900-13. doi: 10.1172/JCI64102. Epub 2012 Oct 15. J Clin Invest. 2012. PMID: 23064363 Free PMC article.
-
Maternal Diabetes and Fetal Programming Toward Neurological Diseases: Beyond Neural Tube Defects.Front Endocrinol (Lausanne). 2018 Nov 13;9:664. doi: 10.3389/fendo.2018.00664. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30483218 Free PMC article. Review.
-
Nutritional programming of hypothalamic development: critical periods and windows of opportunity.Int J Obes Suppl. 2012 Dec;2(Suppl 2):S19-24. doi: 10.1038/ijosup.2012.17. Epub 2012 Dec 11. Int J Obes Suppl. 2012. PMID: 27152149 Free PMC article. Review.
-
Development, brain plasticity and reward: early high-fat diet exposure confers vulnerability to obesity-view from the chair.Int J Obes Suppl. 2012 Dec;2(Suppl 2):S3-6. doi: 10.1038/ijosup.2012.14. Epub 2012 Dec 11. Int J Obes Suppl. 2012. PMID: 27152151 Free PMC article.
References
-
- Whitaker RC, Pepe MS, Seidel KD, Wright JA, Knopp RH. 1998. Gestational diabetes and the risk of offspring obesity. Pediatrics 101:e9. - PubMed
-
- Silverman BL, Metzger BE, Cho NH, Loeb CA. 1995. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 18:611–617 - PubMed
-
- Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. 1988. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes 37:622–628 - PubMed
-
- Berenson GS, Bao W, Srinivasan SR. 1996. Abnormal characteristics of young offspring of parents with noninsulin-dependent diabetes mellitus. The Bogalusa heart study. Am J Epidemiol 144:962–967 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

