Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage
- PMID: 21862613
- PMCID: PMC3199005
- DOI: 10.1210/en.2011-1326
Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage
Abstract
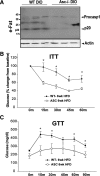
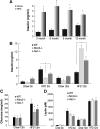
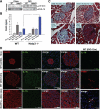
Clinical evidence that the blockade of IL-1β in type-2 diabetic patients improves glycemia is indicative of an autoinflammatory mechanism that may trigger adiposity-driven pancreatic damage. IL-1β is a key contributor to the obesity-induced inflammation and subsequent insulin resistance, pancreatic β-cell dysfunction, and the onset of type 2 diabetes. Our previous studies demonstrated that the ceramides activate the Nod-like receptor family, pyrin domain containing 3 (Nlrp3) inflammasome to cause the generation of mature IL-1β and ablation of the Nlrp3 inflammasome in diet-induced obesity improves insulin signaling. However, it remains unclear whether the posttranslational processing of active IL-1β in pancreas is regulated by the NLRP3 inflammasome or whether the alternate mechanisms play a dominant role in chronic obesity-induced pancreatic β-cell exhaustion. Here we show that loss of ASC, a critical adaptor required for the assembly of the NLRP3 and absent in melanoma 2 inflammasome substantially improves the insulin action. Surprisingly, despite lower insulin resistance in the chronically obese NLRP3 and ASC knockout mice, the insulin levels were substantially higher when the inflammasome pathway was eliminated. The obesity-induced increase in maturation of pancreatic IL-1β and pancreatic islet fibrosis was dependent on the NLRP3 inflammasome activation. Furthermore, elimination of NLRP3 inflammasome protected the pancreatic β-cells from cell death caused by long-term high-fat feeding during obesity with significant increase in the size of the islets of Langerhans. Collectively, this study provides direct in vivo evidence that activation of the NLRP3 inflammasome in diet-induced obesity is a critical trigger in causing pancreatic damage and is an important mechanism of progression toward type 2 diabetes.
Figures

Comment in
-
Inflammation as a sensor of metabolic stress in obesity and type 2 diabetes.Endocrinology. 2011 Nov;152(11):4005-6. doi: 10.1210/en.2011-1691. Endocrinology. 2011. PMID: 22021196 No abstract available.
References
-
- Donath MY, Shoelson SE. 2011. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11:8–107 - PubMed
-
- Dinarello CA, Donath MY, Mandrup-Poulsen T. 2010. Role of IL-1β in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 17:314–321 - PubMed
-
- Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, Baunbjerg Nielsen D, Spohr C, Kober L, Vaag A, Torp-Pedersen C. 2005. Metabolic and vascular effects of tumor necrosis factor-α blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res 42:517–525 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

