Mitochondrial dysfunction and insulin resistance from the outside in: extracellular matrix, the cytoskeleton, and mitochondria
- PMID: 21862724
- PMCID: PMC3214002
- DOI: 10.1152/ajpendo.00363.2011
Mitochondrial dysfunction and insulin resistance from the outside in: extracellular matrix, the cytoskeleton, and mitochondria
Abstract
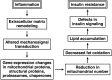
Insulin resistance in skeletal muscle is a prominent feature of obesity and type 2 diabetes. The association between mitochondrial changes and insulin resistance is well known. More recently, there is growing evidence of a relationship between inflammation, extracellular remodeling, and insulin resistance. The intent of this review is to propose a potentially novel mechanism for the development of insulin resistance, focusing on the underappreciated connections among inflammation, extracellular remodeling, cytoskeletal interactions, mitochondrial function, and insulin resistance in human skeletal muscle. Several sources of inflammation, including expansion of adipose tissue resulting in increased lipolysis and alterations in pro- and anti-inflammatory cytokines, contribute to the insulin resistance observed in obesity and type 2 diabetes. In the experimental model of lipid oversupply, an inflammatory response in skeletal muscle leads to altered expression extracellular matrix-related genes as well as nuclear encoded mitochondrial genes. A similar pattern also is observed in "naturally" occurring insulin resistance in muscle of obese nondiabetic individuals and patients with type 2 diabetes mellitus. More recently, alterations in proteins (including α-actinin-2, desmin, proteasomes, and chaperones) involved in muscle structure and function have been observed in insulin-resistant muscle. Some of these cytoskeletal proteins are mechanosignal transducers that allow muscle fibers to sense contractile activity and respond appropriately. The ensuing alterations in expression of genes coding for mitochondrial proteins and cytoskeletal proteins may contribute to the mitochondrial changes observed in insulin-resistant muscle. These changes in turn may lead to a reduction in fat oxidation and an increase in intramyocellular lipid, which contributes to the defects in insulin signaling in insulin resistance.
Figures
References
-
- Bajaj M, Medina-Navarro R, Suraamornkul S, Meyer C, DeFronzo RA, Mandarino LJ. Paradoxical changes in muscle gene expression in insulin-resistant subjects after sustained reduction in plasma free fatty acid concentration. Diabetes 56: 743–752, 2007 - PubMed
-
- Berman Y, North KN. A gene for speed: the emerging role of alpha-actinin-3 in muscle metabolism. Physiology (Bethesda) 25: 250–259, 2010 - PubMed
-
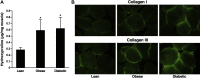
- Berria R, Wang L, Richardson DK, Finlayson J, Belfort R, Pratipanawatr T, De Filippis EA, Kashyap S, Mandarino LJ. Increased collagen content in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 290: E560–E565, 2006 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

