Model of myosin node aggregation into a contractile ring: the effect of local alignment
- PMID: 21862839
- PMCID: PMC3180958
- DOI: 10.1088/0953-8984/23/37/374103
Model of myosin node aggregation into a contractile ring: the effect of local alignment
Abstract
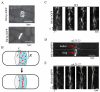
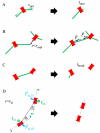
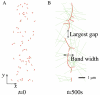
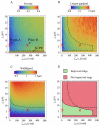
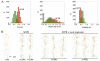
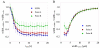
Actomyosin bundles frequently form through aggregation of membrane-bound myosin clusters. One such example is the formation of the contractile ring in fission yeast from a broad band of cortical nodes. Nodes are macromolecular complexes containing several dozens of myosin-II molecules and a few formin dimers. The condensation of a broad band of nodes into the contractile ring has been previously described by a search, capture, pull and release (SCPR) model. In SCPR, a random search process mediated by actin filaments nucleated by formins leads to transient actomyosin connections among nodes that pull one another into a ring. The SCPR model reproduces the transport of nodes over long distances and predicts observed clump-formation instabilities in mutants. However, the model does not generate transient linear elements and meshwork structures as observed in some wild-type and mutant cells during ring assembly. As a minimal model of node alignment, we added short-range aligning forces to the SCPR model representing currently unresolved mechanisms that may involve structural components, cross-linking and bundling proteins. We studied the effect of the local node alignment mechanism on ring formation numerically. We varied the new parameters and found viable rings for a realistic range of values. Morphologically, transient structures that form during ring assembly resemble those observed in experiments with wild-type and cdc25-22 cells. Our work supports a hierarchical process of ring self-organization involving components drawn together from distant parts of the cell followed by progressive stabilization.
Figures


References
-
- Liverpool TB, Marchetti MC. Phys. Rev. Lett. 2003;90:138102. - PubMed
-
- Cytrynbaum EN, Rodionov V, Mogilner A. J. Cell Sci. 2004;117:1381–97. - PubMed
-
- Kruse K, Joanny JF, Julicher F, Prost J, Sekimoto K. Phys. Rev. Lett. 2004;92:078101. - PubMed
-
- Kierfeld J, Khne T, Lipowsky R. Phys. Rev. Lett. 2005;95:038102. - PubMed
-
- Backouche F, Haviv L, Groswasser D, Bernheim-Groswasser A. Phys. Biol. 2006;3:264–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
