A truncated form of CD9-partner 1 (CD9P-1), GS-168AT2, potently inhibits in vivo tumour-induced angiogenesis and tumour growth
- PMID: 21863033
- PMCID: PMC3185932
- DOI: 10.1038/bjc.2011.303
A truncated form of CD9-partner 1 (CD9P-1), GS-168AT2, potently inhibits in vivo tumour-induced angiogenesis and tumour growth
Abstract
Background: Tetraspanins are transmembrane proteins known to contribute to angiogenesis. CD9 partner-1 (CD9P-1/EWI-F), a glycosylated type 1 transmembrane immunoglobulin, is a member of the tetraspanin web, but its role in angiogenesis remains to be elucidated.
Methods: We measured the expression of CD9P-1 under angiogenic and angiostatic conditions, and the influence of its knockdown onto capillary structures formation by human endothelial cells (hECs). A truncated form of CDP-1, GS-168AT2, was produced and challenged vs hEC proliferation, migration and capillaries' formation. Its association with CD9P-1, CD9, CD81 and CD151 and the expressions of these later at hEC surface were analysed. Finally, its effects onto in vivo tumour-induced angiogenesis and tumour growth were investigated.
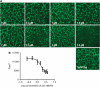
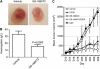
Results: Vascular endothelial growth factor (VEGF)-induced capillary tube-like formation was inhibited by tumour necrosis factor α and was associated with a rise in CD9P-1 mRNA expression (P<0.05); accordingly, knockdown of CD9P-1 inhibited VEGF-dependent in vitro angiogenesis. GS-168AT2 dose-dependently inhibited in vitro angiogenesis, hEC migration and proliferation (P<0.05). Co-precipitation experiments suggest that GS-168AT2 corresponds to the sequence by which CD9P-1 physiologically associates with CD81. GS-168AT2 induced the depletion of CD151, CD9 and CD9P-1 from hEC surface, correlating with GS-168AT2 degradation. Finally, in vivo injections of GS-168AT2 inhibited tumour-associated angiogenesis by 53.4±9.5% (P=0.03), and reduced tumour growth of Calu 6 tumour xenografts by 73.9±16.4% (P=0.007) without bodyweight loss.
Conclusion: The truncated form of CD9P-1, GS-168AT2, potently inhibits angiogenesis and cell migration by at least the downregulation of CD151 and CD9, which provides the first evidences for the central role of CD9P-1 in tumour-associated angiogenesis and tumour growth.
Figures


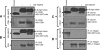


References
-
- Al-Mahmood S (2000) Gene signal international SA, method for identifying novel genes involved in the regulation of angiogenesis, study of said genes and use thereof for therapeutic purposes, US patent no. 6716585
-
- Al-Mahmood S, Colin S, Farhat N, Thorin E, Steverlynck C, Chemtob S (2009) Potent in vivo anti-angiogenic effects of GS-101, an antisense oligonucleotide preventing the expression of Insulin Receptor Substrate-1. J Pharmacol Exper Thera 329: 496–504 - PubMed
-
- Al-Mahmood S, Colin S, Schneider C (2005) Gene signal international SA, genes involved in regulating angiogenesis, pharmaceutical preparations containing same and applications thereof, EP patent no. 1566387
-
- André M, Chambrion C, Charrin S, Soave S, Chaker J, Boucheix C, Rubinstein E, Le Naour F (2009) In situ chemical cross-linking on living cells reveals CD9P-1 cis-oligomer at cell surface. J Proteomics 73: 93–102 - PubMed
-
- Balsari A, Tortoreto M, Besusso D, Petrangolini G, Sfondrini L, Maggi R, Ménard S, Pratesi G (2004) Combination of a CpG oligodeoxynucleotide and a topoisomerase I inhibitor in the therapy of human tumour xenografts. Eur J Cancer 40: 1275–1281 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

