An improved palladium-catalyzed conversion of aryl and vinyl triflates to bromides and chlorides
- PMID: 21863838
- PMCID: PMC3170430
- DOI: 10.1021/ol202098h
An improved palladium-catalyzed conversion of aryl and vinyl triflates to bromides and chlorides
Abstract
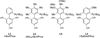
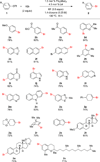
A facile Pd-catalyzed conversion of aryl and vinyl triflates to aryl and vinyl halides (bromides and chlorides) is described. This method allows convenient access to a variety of aryl, heteroaryl, and vinyl halides in good to excellent yields and with greatly simplified conditions relative to our previous report.
© 2011 American Chemical Society
Figures

References
-
- Gribble GW. Acc. Chem. Res. 1998;31:141.
- Gribble GW. Chem. Soc. Rev. 1999;28:335.
- Gribble GW. J. Nat. Prod. 1992;55:1353.
-
-
For selected reviews, see: Knochel P, Dohle W, Gommermann N, Kneisel FF, Kopp F, Korn T, Sapountzis I, Vu VA. Angew. Chem. Int. Ed. 2003;42:4302. Parham WE, Bradsher CK. Acc. Chem. Res. 1982;15:300. Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M. Chem. Rev. 2002;102:1359.
-
-
- Wiley GA, Hershkowitz RL, Rein BM, Chung BC. J. Am. Chem. Soc. 1964;86:964.
- Bay E, Bak DA, Timony PE, Leonebay A. J. Org. Chem. 1990;55:3415.
- Albert A, Clark J. J. Chem. Soc. 1964;1666
- Bendich A, Russell PJ, Fox JJ. J. Am. Chem. Soc. 1954;76:6073.
- Surrey AR, Cutler RA. J. Am. Chem. Soc. 1954;76:1109.
- Bestmann HJ, Schnabel KH. Liebigs Ann. Chem. 1966;698:106.
-
- Wulff WD, Peterson GA, Bauta WE, Chan K-S, Faron KL, Gilbertson SR, Kaesler RW, Yang DC, Murray CK. J. Org. Chem. 1986;51:277.
- Kang H, Facchetti A, Stern CL, Rheingold AL, Kassel WS, Marks TJ. Org. Lett. 2005;7:3721. - PubMed
- Grunewald GL, Seim MR, Regier RC, Criscione KR. Bioorg. Med. Chem. 2007;15:1298. - PMC - PubMed
- Thompson ALS, Kabalka GW, Akula MR, Huffman JW. Synthesis. 2005;547
-
-
For selected reviews, see: Ritter K. Synthesis. 1993;735 Scott WJ, McMurry JE. Acc. Chem. Res. 1988;21:47.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

