Novel anticancer polymeric conjugates of activated nucleoside analogues
- PMID: 21863885
- PMCID: PMC3200571
- DOI: 10.1021/bc200173e
Novel anticancer polymeric conjugates of activated nucleoside analogues
Abstract
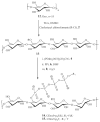
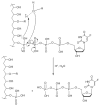
Inherent or therapy-induced drug resistance is a major clinical setback in cancer treatment. The extensive usage of cytotoxic nucleobases and nucleoside analogues in chemotherapy also results in the development of specific mechanisms of drug resistance, such as nucleoside transport or activation deficiencies. These drugs are prodrugs; and being converted into the active mono-, di-, and triphosphates inside cancer cells following administration, they affect nucleic acid synthesis, nucleotide metabolism, or sensitivity to apoptosis. Previously, we actively promoted the idea that the nanodelivery of active nucleotide species, e.g., 5'-triphosphates of nucleoside analogues, can enhance drug efficacy and reduce nonspecific toxicity. In this study, we report the development of a novel type of drug nanoformulations, polymeric conjugates of nucleoside analogues, which are capable of the efficient transport and sustained release of phosphorylated drugs. These drug conjugates have been synthesized, starting from cholesterol-modified mucoadhesive polyvinyl alcohol or biodegradable dextrin, by covalent attachment of nucleoside analogues through a tetraphosphate linker. Association of cholesterol moieties in aqueous media resulted in intramolecular polymer folding and the formation of small nanogel particles containing 0.5 mmol/g of a 5'-phosphorylated nucleoside analogue, e.g., 5-fluoro-2'-deoxyuridine (floxuridine, FdU), an active metabolite of anticancer drug 5-fluorouracyl (5-FU). The polymeric conjugates demonstrated rapid enzymatic release of floxuridine 5'-phosphate and much slower drug release under hydrolytic conditions (pH 1.0-7.4). Among the panel of cancer cell lines, all studied polymeric FdU-conjugates demonstrated an up to 50× increased cytotoxicity in human prostate cancer PC-3, breast cancer MCF-7, and MDA-MB-231 cells, and more than 100× higher efficacy against cytarabine-resistant human T-lymphoma (CEM/araC/8) and gemcitabine-resistant follicular lymphoma (RL7/G) cells as compared to free drugs. In the initial in vivo screening, both PC-3 and RL7/G subcutaneous tumor xenograft models showed enhanced sensitivity to sustained drug release from polymeric FdU-conjugate after peritumoral injections and significant tumor growth inhibition. All these data demonstrate a remarkable clinical potential of novel polymeric conjugates of phosphorylated nucleoside analogues, especially as new therapeutic agents against drug-resistant tumors.
Figures








References
-
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. - PubMed
-
- Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002;3:415–424. - PubMed
-
- Galmarini CM, Thomas X, Calvo F, Rousselot P, Rabilloud M, El Jaffari A, Cros E, Dumontet C. In vivo mechanisms of resistance to cytarabine in acute myeloid leukaemia. Br J Haematol. 2002;117:860–868. - PubMed
-
- Ward JL, Sherali A, Mo ZP, Tse CM. Kinetic and pharmacological properties of cloned human equilibrative nucleoside transporters, ENT1 and ENT2, stably expressed in nucleoside transporter-deficient PK15 cells. Ent2 exhibits a low affinity for guanosine and cytidine but a high affinity for inosine. J Biol Chem. 2000;275:8375–8381. - PubMed
-
- Crawford CR, Ng CY, Belt JA. Isolation and characterization of an L1210 cell line retaining the sodium-dependent carrier cif as its sole nucleoside transport activity. J Biol Chem. 1990;265:13730–13734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

