Directed intermixing in multicomponent self-assembling biomaterials
- PMID: 21863894
- PMCID: PMC3190078
- DOI: 10.1021/bm200763y
Directed intermixing in multicomponent self-assembling biomaterials
Abstract
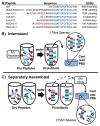
The noncovalent coassembly of multiple different peptides can be a useful route for producing multifunctional biomaterials. However, to date, such materials have almost exclusively been investigated as homogeneous self-assemblies, having functional components uniformly distributed throughout their supramolecular structures. Here we illustrate control over the intermixing of multiple different self-assembling peptides, in turn providing a simple but powerful means for modulating these materials' mechanical and biological properties. In β-sheet fibrillizing hydrogels, significant increases in stiffening could be achieved using heterobifunctional cross-linkers by sequestering peptides bearing different reactive groups into distinct populations of fibrils, thus favoring interfibril cross-linking. Further, by specifying the intermixing of RGD-bearing peptides in 2-D and 3-D self-assemblies, the growth of HUVECs and NIH 3T3 cells could be significantly modulated. This approach may be immediately applicable toward a wide variety of self-assembling systems that form stable supramolecular structures.
Figures





Similar articles
-
Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering.J Am Chem Soc. 2019 Mar 27;141(12):4886-4899. doi: 10.1021/jacs.8b13363. Epub 2019 Mar 12. J Am Chem Soc. 2019. PMID: 30830776
-
Supramolecular hydrogels inspired by collagen for tissue engineering.Org Biomol Chem. 2010 Jul 21;8(14):3267-71. doi: 10.1039/c002609c. Epub 2010 May 26. Org Biomol Chem. 2010. PMID: 20502821
-
Cross-Linking Approaches to Tuning the Mechanical Properties of Peptide π-Electron Hydrogels.Bioconjug Chem. 2017 Mar 15;28(3):751-759. doi: 10.1021/acs.bioconjchem.6b00593. Epub 2016 Dec 1. Bioconjug Chem. 2017. PMID: 28292179
-
Advances in bioactive hydrogels to probe and direct cell fate.Annu Rev Chem Biomol Eng. 2012;3:421-44. doi: 10.1146/annurev-chembioeng-062011-080945. Epub 2012 Apr 17. Annu Rev Chem Biomol Eng. 2012. PMID: 22524507 Review.
-
Design Strategies of Stimuli-Responsive Supramolecular Hydrogels Relying on Structural Analyses and Cell-Mimicking Approaches.Acc Chem Res. 2017 Apr 18;50(4):740-750. doi: 10.1021/acs.accounts.7b00070. Epub 2017 Mar 2. Acc Chem Res. 2017. PMID: 28252940 Review.
Cited by
-
Photodynamic control of bioactivity in a nanofiber matrix.ACS Nano. 2012 Dec 21;6(12):10776-85. doi: 10.1021/nn304101x. Epub 2012 Nov 15. ACS Nano. 2012. PMID: 23153342 Free PMC article.
-
Self-assembling peptide scaffolds for regenerative medicine.Chem Commun (Camb). 2012 Jan 4;48(1):26-33. doi: 10.1039/c1cc15551b. Epub 2011 Nov 14. Chem Commun (Camb). 2012. PMID: 22080255 Free PMC article. Review.
-
Multicomponent peptide assemblies.Chem Soc Rev. 2018 May 21;47(10):3659-3720. doi: 10.1039/c8cs00115d. Chem Soc Rev. 2018. PMID: 29697126 Free PMC article. Review.
-
Enabling sublingual peptide immunization with molecular self-assemblies.Biomaterials. 2020 May;241:119903. doi: 10.1016/j.biomaterials.2020.119903. Epub 2020 Feb 24. Biomaterials. 2020. PMID: 32143059 Free PMC article.
-
Low Molecular Weight Supramolecular Hydrogels for Sustained and Localized In Vivo Drug Delivery.ACS Appl Bio Mater. 2019 Apr 4;2(5):2116-2124. doi: 10.1021/acsabm.9b00125. Epub 2019 May 20. ACS Appl Bio Mater. 2019. PMID: 34136760 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

