Divergent effects of compounds on the hydrolysis and transpeptidation reactions of γ-glutamyl transpeptidase
- PMID: 21864033
- PMCID: PMC3407035
- DOI: 10.3109/14756366.2011.597748
Divergent effects of compounds on the hydrolysis and transpeptidation reactions of γ-glutamyl transpeptidase
Abstract
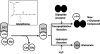
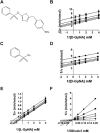
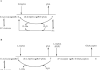
A novel class of inhibitors of the enzyme γ-glutamyl transpeptidase (GGT) were evaluated. The analog OU749 was shown previously to be an uncompetitive inhibitor of the GGT transpeptidation reaction. The data in this study show that it is an equally potent uncompetitive inhibitor of the hydrolysis reaction, the primary reaction catalyzed by GGT in vivo. A series of structural analogs of OU749 were evaluated. For many of the analogs, the potency of the inhibition differed between the hydrolysis and transpeptidation reactions, providing insight into the malleability of the active site of the enzyme. Analogs with electron withdrawing groups on the benzosulfonamide ring, accelerated the hydrolysis reaction, but inhibited the transpeptidation reaction by competing with a dipeptide acceptor. Several of the OU749 analogs inhibited the transpeptidation reaction by slow onset kinetics, similar to acivicin. Further development of inhibitors of the GGT hydrolysis reaction is necessary to provide new therapeutic compounds.
Figures

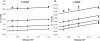

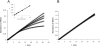
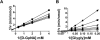
References
-
- Hanigan MH. gamma-Glutamyl transpeptidase, a glutathionase: its expression and function in carcinogenesis. Chem Biol Interact. 1998;111-112:333–342. - PubMed
-
- Hanigan MH, Frierson HF., Jr. Immunohistochemical detection of gamma-glutamyl transpeptidase in normal human tissue. J Histochem Cytochem. 1996;44:1101–1108. - PubMed
-
- Hanigan MH, Frierson HF, Jr., Swanson PE, De Young BR. Altered expression of gamma-glutamyl transpeptidase in human tumors. Hum Pathol. 1999;30:300–305. - PubMed
-
- Ruoso P, Hedley DW. Inhibition of gamma-glutamyl transpeptidase activity decreases intracellular cysteine levels in cervical carcinoma. Cancer Chemother Pharmacol. 2004;54:49–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
