Azithromycin for prevention of exacerbations of COPD
- PMID: 21864166
- PMCID: PMC3220999
- DOI: 10.1056/NEJMoa1104623
Azithromycin for prevention of exacerbations of COPD
Erratum in
- N Engl J Med. 2012 Apr 5;366(14):1356
Abstract
Background: Acute exacerbations adversely affect patients with chronic obstructive pulmonary disease (COPD). Macrolide antibiotics benefit patients with a variety of inflammatory airway diseases.
Methods: We performed a randomized trial to determine whether azithromycin decreased the frequency of exacerbations in participants with COPD who had an increased risk of exacerbations but no hearing impairment, resting tachycardia, or apparent risk of prolongation of the corrected QT interval.
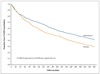
Results: A total of 1577 subjects were screened; 1142 (72%) were randomly assigned to receive azithromycin, at a dose of 250 mg daily (570 participants), or placebo (572 participants) for 1 year in addition to their usual care. The rate of 1-year follow-up was 89% in the azithromycin group and 90% in the placebo group. The median time to the first exacerbation was 266 days (95% confidence interval [CI], 227 to 313) among participants receiving azithromycin, as compared with 174 days (95% CI, 143 to 215) among participants receiving placebo (P<0.001). The frequency of exacerbations was 1.48 exacerbations per patient-year in the azithromycin group, as compared with 1.83 per patient-year in the placebo group (P=0.01), and the hazard ratio for having an acute exacerbation of COPD per patient-year in the azithromycin group was 0.73 (95% CI, 0.63 to 0.84; P<0.001). The scores on the St. George's Respiratory Questionnaire (on a scale of 0 to 100, with lower scores indicating better functioning) improved more in the azithromycin group than in the placebo group (a mean [±SD] decrease of 2.8±12.8 vs. 0.6±11.4, P=0.004); the percentage of participants with more than the minimal clinically important difference of -4 units was 43% in the azithromycin group, as compared with 36% in the placebo group (P=0.03). Hearing decrements were more common in the azithromycin group than in the placebo group (25% vs. 20%, P=0.04).
Conclusions: Among selected subjects with COPD, azithromycin taken daily for 1 year, when added to usual treatment, decreased the frequency of exacerbations and improved quality of life but caused hearing decrements in a small percentage of subjects. Although this intervention could change microbial resistance patterns, the effect of this change is not known. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT00325897.).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures


Comment in
-
Preventing exacerbations of COPD--advice from Hippocrates.N Engl J Med. 2011 Aug 25;365(8):753-4. doi: 10.1056/NEJMe1106979. N Engl J Med. 2011. PMID: 21864170 No abstract available.
-
Azithromycin for prevention of exacerbations of COPD.N Engl J Med. 2011 Dec 8;365(23):2234; author reply 2236. doi: 10.1056/NEJMc1111248. N Engl J Med. 2011. PMID: 22150045 No abstract available.
-
Azithromycin for prevention of exacerbations of COPD.N Engl J Med. 2011 Dec 8;365(23):2234-5; author reply 2236. doi: 10.1056/NEJMc1111248. N Engl J Med. 2011. PMID: 22150046 No abstract available.
-
Azithromycin for prevention of exacerbations of COPD.N Engl J Med. 2011 Dec 8;365(23):2235; author reply 2236. doi: 10.1056/NEJMc1111248. N Engl J Med. 2011. PMID: 22150047 No abstract available.
-
Azithromycin for prevention of exacerbations of COPD.N Engl J Med. 2011 Dec 8;365(23):2236-6; author reply 2236-7. doi: 10.1056/NEJMc1111248. N Engl J Med. 2011. PMID: 22150048 No abstract available.
-
Azithromycin once daily for 1 year reduced acute COPD exacerbations.Ann Intern Med. 2012 Jan 17;156(2):JC1-10. doi: 10.7326/0003-4819-156-2-201201170-02010. Ann Intern Med. 2012. PMID: 22250172 No abstract available.
-
New treatments for idiopathic pulmonary fibrosis, empyema, and chronic obstructive pulmonary disease.Am J Respir Crit Care Med. 2012 Mar 15;185(6):680-1. doi: 10.1164/rccm.201110-1871RR. Am J Respir Crit Care Med. 2012. PMID: 22422903 No abstract available.
-
Consider adding this drug to fight COPD that's severe.J Fam Pract. 2012 Jul;61(7):414-6. J Fam Pract. 2012. PMID: 22754891 Free PMC article.
References
-
- Strassels SA, Smith DH, Sullivan SD, Mahajan PS. The costs of treating COPD in the United States. Chest. 2001;119:344–352. - PubMed
-
- Andersson F, Borg S, Jansson SA, et al. The costs of exacerbations in chronic obstructive pulmonary disease (COPD) Respir Med. 2002;96:700–708. - PubMed
-
- Druss BG, Marcus SC, Olfson M, Pincus HA. The most expensive medical conditions in America. Health Aff (Millwood) 2002;21:105–111. - PubMed
-
- Miller JD, Foster T, Boulanger L, et al. Direct costs of COPD in the U.S.: an analysis of Medical Expenditure Panel Survey (MEPS) data. COPD. 2005;2:311–318. - PubMed
-
- Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive. 144:894–903. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL074416/HL/NHLBI NIH HHS/United States
- 1U10-HL074431/HL/NHLBI NIH HHS/United States
- M01 RR16500/RR/NCRR NIH HHS/United States
- U10 HL074428/HL/NHLBI NIH HHS/United States
- HL074407/HL/NHLBI NIH HHS/United States
- HL074418/HL/NHLBI NIH HHS/United States
- HL074408/HL/NHLBI NIH HHS/United States
- U10 HL074409/HL/NHLBI NIH HHS/United States
- U10 HL074431/HL/NHLBI NIH HHS/United States
- HL074439/HL/NHLBI NIH HHS/United States
- U10 HL074424/HL/NHLBI NIH HHS/United States
- M01 RR00425/RR/NCRR NIH HHS/United States
- M01 RR00056/RR/NCRR NIH HHS/United States
- 1U10-HL074416/HL/NHLBI NIH HHS/United States
- M01 RR00051/RR/NCRR NIH HHS/United States
- HL074428/HL/NHLBI NIH HHS/United States
- U10 HL074439/HL/NHLBI NIH HHS/United States
- M01 RR02635/RR/NCRR NIH HHS/United States
- U10 HL074408/HL/NHLBI NIH HHS/United States
- HL074422/HL/NHLBI NIH HHS/United States
- U10 HL074422/HL/NHLBI NIH HHS/United States
- HL074441/HL/NHLBI NIH HHS/United States
- U10 HL074407/HL/NHLBI NIH HHS/United States
- U10 HL074441/HL/NHLBI NIH HHS/United States
- K23 HL089353/HL/NHLBI NIH HHS/United States
- 1U10- HL074424/HL/NHLBI NIH HHS/United States
- U10 HL074418/HL/NHLBI NIH HHS/United States
- HL074409/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
