Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors
- PMID: 21864401
- PMCID: PMC3170653
- DOI: 10.1186/1471-2407-11-371
Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors
Abstract
Background: Betulinic acid (BA) inhibits growth of several cancer cell lines and tumors and the effects of BA have been attributed to its mitochondriotoxicity and inhibition of multiple pro-oncogenic factors. Previous studies show that BA induces proteasome-dependent degradation of specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 in prostate cancer cells and this study focused on the mechanism of action of BA in colon cancer cells.
Methods: The effects of BA on colon cancer cell proliferation and apoptosis and tumor growth in vivo were determined using standardized assays. The effects of BA on Sp proteins and Sp-regulated gene products were analyzed by western blots, and real time PCR was used to determine microRNA-27a (miR-27a) and ZBTB10 mRNA expression.
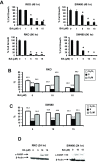
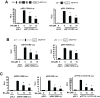
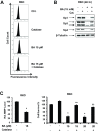
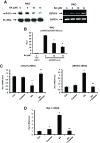
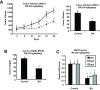
Results: BA inhibited growth and induced apoptosis in RKO and SW480 colon cancer cells and inhibited tumor growth in athymic nude mice bearing RKO cells as xenograft. BA also decreased expression of Sp1, Sp3 and Sp4 transcription factors which are overexpressed in colon cancer cells and decreased levels of several Sp-regulated genes including survivin, vascular endothelial growth factor, p65 sub-unit of NFκB, epidermal growth factor receptor, cyclin D1, and pituitary tumor transforming gene-1. The mechanism of action of BA was dependent on cell context, since BA induced proteasome-dependent and proteasome-independent downregulation of Sp1, Sp3 and Sp4 in SW480 and RKO cells, respectively. In RKO cells, the mechanism of BA-induced repression of Sp1, Sp3 and Sp4 was due to induction of reactive oxygen species (ROS), ROS-mediated repression of microRNA-27a, and induction of the Sp repressor gene ZBTB10.
Conclusions: These results suggest that the anticancer activity of BA in colon cancer cells is due, in part, to downregulation of Sp1, Sp3 and Sp4 transcription factors; however, the mechanism of this response is cell context-dependent.
Figures

References
-
- Tomlinson IP, Hampson R, Karran P, Bodmer WF. DNA mismatch repair in lymphoblastoid cells from hereditary non-polyposis colorectal cancer (HNPCC) patients is normal under conditions of rapid cell division and increased mutational load. Mutat Res. 1997;383:177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

