Cross talk between focal adhesion kinase and cadherins: role in regulating endothelial barrier function
- PMID: 21864544
- PMCID: PMC3427140
- DOI: 10.1016/j.mvr.2011.08.001
Cross talk between focal adhesion kinase and cadherins: role in regulating endothelial barrier function
Abstract
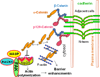
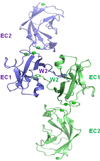
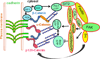
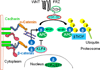
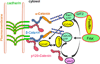
A layer of endothelial cells attached to their underlying matrices by complex transmembrane structures termed focal adhesion (FA) proteins maintains the barrier property of microvascular endothelium. FAs sense the physical properties of the extracellular matrix (ECM) and organize the cytoskeleton accordingly. The close association of adherens junction (AJ) protein, cadherin, with the cytoskeleton is known to be essential in coordinating the appropriate mechanical properties to cell-cell contacts. Recently, it has become clear that a crosstalk exists between focal adhesion kinase (FAK) and cadherin that regulates signaling at intercellular endothelial junctions. This review discusses recent advances in our understanding of the dynamic regulation of the molecular connections between FAK and the cadherin complex and cadherin-catenin-actin interaction-dependent changes as well as the role of small GTPases in endothelial barrier regulation. This review also discusses how a signaling network regulates a range of cellular processes important for barrier function and diseases.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Abercrombie M, Dunn GA. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp Cell Res. 1975;92:57–62. - PubMed
-
- Abercrombie M, et al. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67:359–367. - PubMed
-
- Aberle H, et al. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107(Pt 12):3655–3663. - PubMed
-
- Al-Amoudi A, Frangakis AS. Structural studies on desmosomes. Biochem Soc Trans. 2008;36:181–187. - PubMed
-
- Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113(Pt 8):1319–1334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

