Evaluation of age-related differences in the immunogenicity of a G9 H9N2 influenza vaccine
- PMID: 21864622
- PMCID: PMC3196602
- DOI: 10.1016/j.vaccine.2011.08.044
Evaluation of age-related differences in the immunogenicity of a G9 H9N2 influenza vaccine
Abstract
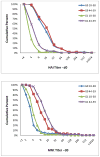
Avian influenza A/H9N2 viruses can infect people and are viruses considered to be a potential pandemic threat. Prior studies with an inactivated G1 clade H9N2 vaccine reported that persons born before 1968 were more likely to have an immune response than younger subjects. We performed a randomized, double-blind trial to evaluate whether immune responses following immunization with an inactivated, unadjuvanted influenza G9 H9N2 vaccine prepared from A/chicken/Hong Kong/G9/97 virus were more frequent in persons born in 1964 or earlier (44-59 years) than in those born in 1970 or later (18-38 years). One hundred twenty one persons were randomized to receive two doses of either 7.5- or 30-mcg of hemagglutinin intramuscularly. Post-vaccination serum antibody responses as measured by hemagglutination inhibition and microneutralization were either similar in the two age cohorts or greater in the younger age group. Persons born before 1968 were not more likely to respond to a G9 H9N2 influenza vaccine than persons born in 1970 or later.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
-
- Lee YJ, Shin JY, Song MS, et al. Continuing evolution of H9 influenza viruses in Korean poultry. Virology. 2007;359:313–23. - PubMed
-
- Peiris M, Yuen KY, Leung CW, et al. Human infection with influenza H9N2. Lancet. 1999;354:916–7. - PubMed
-
- World Health Organization. Responding to the avian influenza pandemic threat. Recommended strategic actions. Geneva: World Health Organization; 2005. Pamphlet no. WHO/CDS/CSR/GIP/2005.8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

