Management of cytoskeleton architecture by molecular chaperones and immunophilins
- PMID: 21864675
- PMCID: PMC3184352
- DOI: 10.1016/j.cellsig.2011.07.023
Management of cytoskeleton architecture by molecular chaperones and immunophilins
Abstract
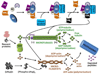
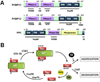
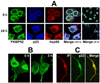
Cytoskeletal structure is continually remodeled to accommodate normal cell growth and to respond to pathophysiological cues. As a consequence, several cytoskeleton-interacting proteins become involved in a variety of cellular processes such as cell growth and division, cell movement, vesicle transportation, cellular organelle location and function, localization and distribution of membrane receptors, and cell-cell communication. Molecular chaperones and immunophilins are counted among the most important proteins that interact closely with the cytoskeleton network, in particular with microtubules and microtubule-associated factors. In several situations, heat-shock proteins and immunophilins work together as a functionally active heterocomplex, although both types of proteins also show independent actions. In circumstances where homeostasis is affected by environmental stresses or due to genetic alterations, chaperone proteins help to stabilize the system. Molecular chaperones facilitate the assembly, disassembly and/or folding/refolding of cytoskeletal proteins, so they prevent aberrant protein aggregation. Nonetheless, the roles of heat-shock proteins and immunophilins are not only limited to solve abnormal situations, but they also have an active participation during the normal differentiation process of the cell and are key factors for many structural and functional rearrangements during this course of action. Cytoskeleton modifications leading to altered localization of nuclear factors may result in loss- or gain-of-function of such factors, which affects the cell cycle and cell development. Therefore, cytoskeletal components are attractive therapeutic targets, particularly microtubules, to prevent pathological situations such as rapidly dividing tumor cells or to favor the process of cell differentiation in other cases. In this review we will address some classical and novel aspects of key regulatory functions of heat-shock proteins and immunophilins as housekeeping factors of the cytoskeletal network.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52.Biomolecules. 2019 Feb 1;9(2):52. doi: 10.3390/biom9020052. Biomolecules. 2019. PMID: 30717249 Free PMC article. Review.
-
Molecular chaperones and the cytoskeleton.J Cell Sci. 1997 Jul;110 ( Pt 13):1431-40. doi: 10.1242/jcs.110.13.1431. J Cell Sci. 1997. PMID: 9224761 Review.
-
A model for the cytoplasmic trafficking of signalling proteins involving the hsp90-binding immunophilins and p50cdc37.Cell Signal. 1999 Dec;11(12):839-51. doi: 10.1016/s0898-6568(99)00064-9. Cell Signal. 1999. PMID: 10659992 Review.
-
Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery.Exp Biol Med (Maywood). 2003 Feb;228(2):111-33. doi: 10.1177/153537020322800201. Exp Biol Med (Maywood). 2003. PMID: 12563018 Review.
-
The role of p23, Hop, immunophilins, and other co-chaperones in regulating Hsp90 function.Methods Mol Biol. 2011;787:45-66. doi: 10.1007/978-1-61779-295-3_4. Methods Mol Biol. 2011. PMID: 21898226
Cited by
-
Sensor potency of the moonlighting enzyme-decorated cytoskeleton: the cytoskeleton as a metabolic sensor.BMC Biochem. 2013 Feb 11;14:3. doi: 10.1186/1471-2091-14-3. BMC Biochem. 2013. PMID: 23398642 Free PMC article.
-
HSP90AB1: Helping the good and the bad.Gene. 2016 Jan 10;575(2 Pt 1):171-86. doi: 10.1016/j.gene.2015.08.063. Epub 2015 Sep 7. Gene. 2016. PMID: 26358502 Free PMC article. Review.
-
Cadmium Highlights Common and Specific Responses of Two Freshwater Sentinel Species, Dreissena polymorpha and Dreissena rostriformis bugensis.Proteomes. 2024 Mar 26;12(2):10. doi: 10.3390/proteomes12020010. Proteomes. 2024. PMID: 38651369 Free PMC article.
-
Novel therapeutic strategies for ischemic heart disease.Pharmacol Res. 2014 Nov;89:36-45. doi: 10.1016/j.phrs.2014.08.004. Epub 2014 Sep 1. Pharmacol Res. 2014. PMID: 25193582 Free PMC article. Review.
-
Proteomics Reveal the Profiles of Color Change in Brunfelsia acuminata Flowers.Int J Mol Sci. 2019 Apr 23;20(8):2000. doi: 10.3390/ijms20082000. Int J Mol Sci. 2019. PMID: 31018626 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

