Direct evidence of phospholipids in gecko footprints and spatula-substrate contact interface detected using surface-sensitive spectroscopy
- PMID: 21865250
- PMCID: PMC3284128
- DOI: 10.1098/rsif.2011.0370
Direct evidence of phospholipids in gecko footprints and spatula-substrate contact interface detected using surface-sensitive spectroscopy
Abstract
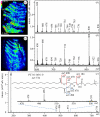
Observers ranging from Aristotle to young children have long marvelled at the ability of geckos to cling to walls and ceilings. Detailed studies have revealed that geckos are 'sticky' without the use of glue or suction devices. Instead, a gecko's stickiness derives from van der Waals interactions between proteinaceous hairs called setae and substrate. Here, we present surprising evidence that although geckos do not use glue, a residue is transferred on surfaces as they walk-geckos leave footprints. Using matrix-free nano-assisted laser desorption-ionization mass spectrometry, we identified the residue as phospholipids with phosphocholine head groups. Moreover, interface-sensitive sum-frequency generation spectroscopy revealed predominantly hydrophobic methyl and methylene groups and the complete absence of water at the contact interface between a gecko toe pad and the substrate. The presence of lipids has never been considered in current models of gecko adhesion. Our analysis of gecko footprints and the toe pad-substrate interface has significant consequences for models of gecko adhesion and by extension, the design of synthetic mimics.
Figures



References
-
- Autumn K., Liang Y. A., Hsieh S. T., Zesch W., Chan W. P., Kenny T. W., Fearing R., Full R. J. 2000. Adhesive force of a single gecko foot-hair. Nature 405, 681–68510.1038/35015073 (doi:10.1038/35015073) - DOI - DOI - PubMed
-
- Autumn K., et al. 2002. Evidence for van der Waals adhesion in gecko setae. Proc. Natl Acad. Sci. USA 99, 12 252–12 25610.1073/pnas.192252799 (doi:10.1073/pnas.192252799) - DOI - DOI - PMC - PubMed
-
- Irschick D. J., Herrel A., Vanhooydonck B. 2006. Whole-organism studies of adhesion in pad-bearing lizards: creative evolutionary solutions to functional problems. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 192, 1169–117710.1007/s00359-006-0145-2 (doi:10.1007/s00359-006-0145-2) - DOI - DOI - PubMed
-
- Arzt E., Gorb S., Spolenak R. 2003. From micro to nano contacts in biological attachment devices. Proc. Natl Acad. Sci. USA 100, 10 603–10 60610.1073/pnas.1534701100 (doi:10.1073/pnas.1534701100) - DOI - DOI - PMC - PubMed
-
- Geim A. K., Dubonos S. V., Grigorieva I. V., Novoselov K. S., Zhukov A. A., Shapoval S. Y. 2003. Microfabricated adhesive mimicking gecko foot-hair. Nat. Mater. 2, 461–46310.1038/nmat917 (doi:10.1038/nmat917) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

