Nanostructural self-assembly of iridescent feather barbules through depletion attraction of melanosomes during keratinization
- PMID: 21865251
- PMCID: PMC3284138
- DOI: 10.1098/rsif.2011.0456
Nanostructural self-assembly of iridescent feather barbules through depletion attraction of melanosomes during keratinization
Abstract
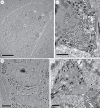
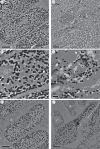
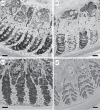
Avian plumage colours are model traits in understanding the evolution of sexually selected ornamental traits. Paradoxically, iridescent structural colours, probably the most dazzling of these traits, remain the most poorly understood. Though some data suggest that expression of bright iridescent plumage colours produced by highly ordered arrays of melanosomes and keratin is condition-dependent, almost nothing is known of their ontogeny and thus of any developmental mechanisms that may be susceptible to perturbation. Here, we use light and electron microscopy to compare the ontogeny of iridescent male and non-iridescent female feathers in blue-black grassquits. Feather barbules of males contain a single layer of melanosomes bounded by a thin layer of keratin-producing blue iridescent colour, while those of females contain disorganized melanosomes and no outer layer. We found that nanostructural organization of male barbules occurs late in development, following death of the barbule cell, and is thus unlikely to be under direct cellular control, contrary to previous suggestions. Rather, organization appears to be caused by entropically driven self-assembly through depletion attraction forces that pin melanosomes to the edge of barbule cells and to one another. These forces are probably stronger in developing barbules of males than of females because their melanosomes are (i) larger, (ii) more densely packed, and (iii) more homogeneously distributed owing to the more consistent shape of barbules during keratinization. These data provide the first proposed developmental pathway for iridescent plumage colours, and suggest that any condition dependence of iridescent barbules is likely driven by factors other than direct metabolic cost.
Figures


Similar articles
-
Ontogeny of an iridescent nanostructure composed of hollow melanosomes.J Morphol. 2015 Apr;276(4):378-84. doi: 10.1002/jmor.20347. Epub 2014 Nov 26. J Morphol. 2015. PMID: 25427951
-
Manakins can produce iridescent and bright feather colours without melanosomes.J Exp Biol. 2016 Jun 15;219(Pt 12):1851-9. doi: 10.1242/jeb.137182. J Exp Biol. 2016. PMID: 27307543
-
Iridescent plumage in satin bowerbirds: structure, mechanisms and nanostructural predictors of individual variation in colour.J Exp Biol. 2006 Jan;209(Pt 2):380-90. doi: 10.1242/jeb.01988. J Exp Biol. 2006. PMID: 16391360
-
Cytochemical and molecular characteristics of the process of cornification during feather morphogenesis.Prog Histochem Cytochem. 2008;43(1):1-69. doi: 10.1016/j.proghi.2008.01.001. Epub 2008 Mar 14. Prog Histochem Cytochem. 2008. PMID: 18394491 Review.
-
Review: cornification, morphogenesis and evolution of feathers.Protoplasma. 2017 May;254(3):1259-1281. doi: 10.1007/s00709-016-1019-2. Epub 2016 Sep 10. Protoplasma. 2017. PMID: 27614891 Review.
Cited by
-
A window on the past: male ornamental plumage reveals the quality of their early-life environment.Proc Biol Sci. 2013 Feb 13;280(1756):20122852. doi: 10.1098/rspb.2012.2852. Print 2013 Apr 7. Proc Biol Sci. 2013. PMID: 23407833 Free PMC article.
-
Avian Pigment Pattern Formation: Developmental Control of Macro- (Across the Body) and Micro- (Within a Feather) Level of Pigment Patterns.Front Cell Dev Biol. 2020 Jul 10;8:620. doi: 10.3389/fcell.2020.00620. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32754601 Free PMC article. Review.
-
Dietary protein level affects iridescent coloration in Anna's hummingbirds, Calypte anna.J Exp Biol. 2012 Aug 15;215(Pt 16):2742-50. doi: 10.1242/jeb.069351. J Exp Biol. 2012. PMID: 22837446 Free PMC article.
-
Heightened condition dependent expression of structural coloration in the faces, but not wings, of male and female flies.Curr Zool. 2021 Oct 18;68(5):600-607. doi: 10.1093/cz/zoab087. eCollection 2022 Oct. Curr Zool. 2021. PMID: 36324536 Free PMC article.
-
Feather Gene Expression Elucidates the Developmental Basis of Plumage Iridescence in African Starlings.J Hered. 2021 Aug 25;112(5):417-429. doi: 10.1093/jhered/esab014. J Hered. 2021. PMID: 33885791 Free PMC article.
References
-
- Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray
-
- Andersson M. 1994. Sexual selection. New Jersey, NJ: Princeton University Press
-
- Searcy W. A., Nowicki S. 2005. The evolution of animal communication: reliability and deception in signaling systems. New Jersey, NJ: Princeton University Press
-
- Prum R. O. 2010. The Lande-Kirkpatrick mechanism is the null model of evolution by intersexual selection: implications for the meaning, honesty, and design in intersexual signals. Evolution 64, 3085–310010.1111/j.1558-5646.2010.01054.x (doi:10.1111/j.1558-5646.2010.01054.x) - DOI - DOI - PubMed
-
- Bennett A. T. D., Cuthill I. C., Partridge J. C., Lunau K. 1997. Ultraviolet plumage colors predict mate preferences in starlings. Proc. Natl Acad. Sci. USA 94, 8618–862110.1073/pnas.94.16.8618 (doi:10.1073/pnas.94.16.8618) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

