Cryoprotection-lyophilization and physical stabilization of rifampicin-loaded flower-like polymeric micelles
- PMID: 21865255
- PMCID: PMC3262430
- DOI: 10.1098/rsif.2011.0414
Cryoprotection-lyophilization and physical stabilization of rifampicin-loaded flower-like polymeric micelles
Abstract
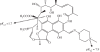
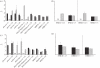
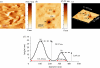
Rifampicin-loaded poly(ε-caprolactone)-b-poly(ethylene glycol)-poly(ε-caprolactone) flower-like polymeric micelles display low aqueous physical stability over time and undergo substantial secondary aggregation. To improve their physical stability, the lyoprotection-lyophilization process was thoroughly characterized. The preliminary cryoprotectant performance of mono- and disaccharides (e.g. maltose, glucose), hydroxypropyl-β-cyclodextrin (HPβCD) and poly(ethylene glycol) (PEG) of different molecular weights was assessed in freeze-thawing assays at -20°C, -80°C and -196°C. The size and size distribution of the micelles at the different stages were measured by dynamic light scattering (DLS). A cryoprotectant factor (f(c)) was determined by taking the ratio between the size immediately after the addition of the cryoprotectant and the size after the preliminary freeze-thawing assay. The benefit of a synergistic cryoprotection by means of saccharide/PEG mixtures was also assessed. Glucose (1 : 20), maltose (1 : 20), HPβCD (1 : 5) and glucose or maltose mixtures with PEG3350 (1 : 20) (copolymer:cryoprotectant weight ratio) were the most effective systems to protect 1 per cent micellar systems. Conversely, only HPβCD (1 : 5) cryoprotected more concentrated drug-loaded micelles (4% and 6%). Then, those micelle/cryoprotectant systems that displayed f(c) values smaller than 2 were freeze-dried. The morphology of freeze-dried powders was characterized by scanning electron microscopy and atomic force microscopy and the residual water content analysed by the Karl Fisher method. The HPβCD-added lyophilisates were brittle porous cakes (residual water was between 0.8% and 3%), easily redispersable in water to form transparent systems with a minimal increase in the micellar size, as determined by DLS.
Figures

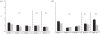

Similar articles
-
Investigations on the lyophilisation of MPEG-hexPLA micelle based pharmaceutical formulations.Eur J Pharm Sci. 2010 Apr 16;40(1):38-47. doi: 10.1016/j.ejps.2010.02.006. Epub 2010 Feb 23. Eur J Pharm Sci. 2010. PMID: 20184955
-
Molecular implications in the nanoencapsulation of the anti-tuberculosis drug rifampicin within flower-like polymeric micelles.Colloids Surf B Biointerfaces. 2010 Sep 1;79(2):467-79. doi: 10.1016/j.colsurfb.2010.05.016. Colloids Surf B Biointerfaces. 2010. PMID: 20627665
-
Influence of Lyophilization and Cryoprotection on the Stability and Morphology of Drug-Loaded Poly(ethylene glycol-b-ε-caprolactone) Micelles.Polymers (Basel). 2023 Apr 21;15(8):1974. doi: 10.3390/polym15081974. Polymers (Basel). 2023. PMID: 37112121 Free PMC article.
-
Physical stability and lyophilization of poly(epsilon-caprolactone)-b-poly(ethyleneglycol)-b-poly(epsilon-caprolactone) micelles.J Nanosci Nanotechnol. 2006 Sep-Oct;6(9-10):3032-9. doi: 10.1166/jnn.2006.432. J Nanosci Nanotechnol. 2006. PMID: 17048515
-
Polymeric micelles from poly(ethylene glycol)-poly(amino acid) block copolymer for drug and gene delivery.J R Soc Interface. 2009 Jun 6;6 Suppl 3(Suppl 3):S325-39. doi: 10.1098/rsif.2008.0547.focus. Epub 2009 Apr 1. J R Soc Interface. 2009. PMID: 19364722 Free PMC article. Review.
Cited by
-
Preparation of blueberry anthocyanin liposomes and changes of vesicle properties, physicochemical properties, in vitro release, and antioxidant activity before and after chitosan modification.Food Sci Nutr. 2021 Dec 2;10(1):75-87. doi: 10.1002/fsn3.2649. eCollection 2022 Jan. Food Sci Nutr. 2021. PMID: 35035911 Free PMC article.
-
Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities.Nanomaterials (Basel). 2020 Apr 28;10(5):847. doi: 10.3390/nano10050847. Nanomaterials (Basel). 2020. PMID: 32354008 Free PMC article. Review.
-
Folding graft copolymer with pendant drug segments for co-delivery of anticancer drugs.Biomaterials. 2014 Aug;35(25):7194-203. doi: 10.1016/j.biomaterials.2014.05.004. Epub 2014 May 27. Biomaterials. 2014. PMID: 24875756 Free PMC article.
-
Synthesis of non-toxic, biocompatible, and colloidal stable silver nanoparticle using egg-white protein as capping and reducing agents for sustainable antibacterial application.RSC Adv. 2018 Jun 25;8(41):23213-23229. doi: 10.1039/c8ra03649g. eCollection 2018 Jun 21. RSC Adv. 2018. Retraction in: RSC Adv. 2022 Jun 17;12(28):18040. doi: 10.1039/d2ra90063g. PMID: 35540173 Free PMC article. Retracted.
-
Poly(ethylene glycol)-b-poly(epsilon-caprolactone) nanoparticles as a platform for the improved oral delivery of cannabidiol.Drug Deliv Transl Res. 2023 Dec;13(12):3192-3203. doi: 10.1007/s13346-023-01380-1. Epub 2023 Jun 21. Drug Deliv Transl Res. 2023. PMID: 37341881
References
-
- Kaufmann S. H., McMichael A. J. 2005. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat. Med. 11, S33.10.1038/nm1221 (doi:10.1038/nm1221) - DOI - DOI - PMC - PubMed
-
- Frieden T. R., Sterling T. R., Munsiff S. S., Watt C. J., Dye C. 2003. Tuberculosis. Lancet 362, 887.10.1016/S0140-6736(03)14333-4 (doi:10.1016/S0140-6736(03)14333-4) - DOI - DOI - PubMed
-
- Ginsberg A. M. 2010. Drugs in development for tuberculosis. Drugs 70, 2201.10.2165/11538170-000000000-00000 (doi:10.2165/11538170-000000000-00000) - DOI - DOI - PubMed
-
- Sharma M., Thapaliya H. P. 2009. Tuberculosis—the disease of poverty. St Xavieŕs J. Sci. 1, 1–9
-
- Jain S. K., Lamichhane G., Nimmagadda S., Pomper M. G., Bishai W. R. 2008. Antibiotic treatment of tuberculosis: old problems, new solutions. Microbe 3, 285–292
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

