A role for heterochromatin protein 1γ at human telomeres
- PMID: 21865325
- PMCID: PMC3175717
- DOI: 10.1101/gad.17325211
A role for heterochromatin protein 1γ at human telomeres
Abstract
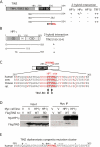
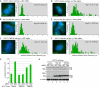
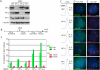
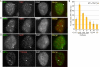
Human telomere function is mediated by shelterin, a six-subunit complex that is required for telomere replication, protection, and cohesion. TIN2, the central component of shelterin, has binding sites to three subunits: TRF1, TRF2, and TPP1. Here we identify a fourth partner, heterochromatin protein 1γ (HP1γ), that binds to a conserved canonical HP1-binding motif, PXVXL, in the C-terminal domain of TIN2. We show that HP1γ localizes to telomeres in S phase, where it is required to establish/maintain cohesion. We further demonstrate that the HP1-binding site in TIN2 is required for sister telomere cohesion and can impact telomere length maintenance by telomerase. Remarkably, the PTVML HP1-binding site is embedded in the recently identified cluster of mutations in TIN2 that gives rise to dyskeratosis congenita (DC), an inherited bone marrow failure syndrome caused by defects in telomere maintenance. We show that DC-associated mutations in TIN2 abrogate binding to HP1γ and that DC patient cells are defective in sister telomere cohesion. Our data indicate a novel requirement for HP1γ in the establishment/maintenance of cohesion at human telomeres and, furthermore, may provide insight into the mechanism of pathogenesis in TIN2-mediated DC.
Figures
References
-
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120–124 - PubMed
-
- Bartel PL, Chien C, Sternglanz R, Fields S 1993. Using the two-hybrid system to detect protein–protein interaction. In Cellular interactions in development: a practical approach (ed. Harley DA), pp. 153–179 IRL Press, Oxford
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
