Impaired FcεRI stability, signaling, and effector functions in murine mast cells lacking glycosylphosphatidylinositol-anchored proteins
- PMID: 21865342
- PMCID: PMC3204909
- DOI: 10.1182/blood-2011-02-338053
Impaired FcεRI stability, signaling, and effector functions in murine mast cells lacking glycosylphosphatidylinositol-anchored proteins
Abstract
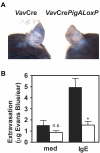
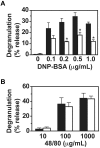
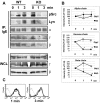
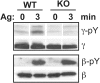
A key event and potential therapeutic target in allergic and asthmatic diseases is signaling by the IgE receptor FcεRI, which depends on its interactions with Src family kinases (SFK). Here we tested the hypothesis that glycosylphosphatidylinositiol-anchored proteins (GPI-AP) are involved in FcεRI signaling, based on previous observations that GPI-AP colocalize with and mediate activation of SFK. We generated mice with a hematopoietic cell-specific GPI-AP deficiency by targeted disruption of the GPI biosynthesis gene PigA. In these mice, IgE-mediated passive cutaneous anaphylaxis was largely abolished. PigA-deficient mast cells cultured from these mice showed impaired degranulation in response to stimulation with IgE and antigen in vitro, despite normal IgE binding and antigen-induced FcεRI aggregation. On stimulation of these cells with IgE and antigen, coprecipitation of the FcεRI α-chain with the γ-chain and β-chain was markedly reduced. As a result, IgE/antigen-induced FcεRI-Lyn association and γ-chain tyrosine phosphorylation were both impaired in PigA-deficient cells. These data provide genetic evidence for an unanticipated key role of GPI-AP in FcεRI interchain interactions and early FcεRI signaling events, necessary for antigen-induced mast cell degranulation.
Figures


References
-
- Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402(6760 Suppl):B24–30. - PubMed
-
- Field KA, Holowka D, Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J Biol Chem. 1997;272(7):4276–4280. - PubMed
-
- Holowka D, Gosse JA, Hammond AT, et al. Lipid segregation and IgE receptor signaling: a decade of progress. Biochim Biophys Acta. 2005;1746(3):252–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

