Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib
- PMID: 21865346
- PMCID: PMC4916618
- DOI: 10.1182/blood-2011-05-355594
Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib
Erratum in
- Blood. 2013 Oct 3;122(14):2524
Abstract
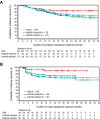
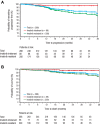
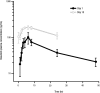
Bosutinib, a dual Src/Abl kinase inhibitor, has shown potent activity against chronic myeloid leukemia (CML). In this phase 1/2 study we evaluated bosutinib in patients with chronic phase imatinib-resistant or imatinib-intolerant CML. Part 1 was a dose-escalation study to determine the recommended starting dose for part 2; part 2 evaluated the efficacy and safety of bosutinib 500 mg once-daily dosing. The study enrolled 288 patients with imatinib-resistant (n = 200) or imatinib-intolerant (n = 88) CML and no other previous kinase inhibitor exposure. At 24 weeks, 31% of patients achieved major cytogenetic response (primary end point). After a median follow-up of 24.2 months, 86% of patients achieved complete hematologic remission, 53% had a major cytogenetic response (41% had a complete cytogenetic response), and 64% of those achieving complete cytogenetic response had a major molecular response. At 2 years, progression-free survival was 79%; overall survival at 2 years was 92%. Responses were seen across Bcr-Abl mutants, except T315I. Bosutinib exhibited an acceptable safety profile; the most common treatment-emergent adverse event was mild/moderate, typically self-limiting diarrhea. Grade 3/4 nonhematologic adverse events (> 2% of patients) included diarrhea (9%), rash (9%), and vomiting (3%). These data suggest bosutinib is effective and tolerable in patients with chronic phase imatinib-resistant or imatinib-intolerant CML. This trial was registered at http://www.clinicaltrials.gov as NCT00261846.
Figures


References
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Chronic Myelogenous Leukemia. V. 2.2009. [Accessed May 12, 2009]. http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf. - PubMed
-
- American Cancer Society. Cancer Facts & Figures, 2010. Atlanta, GA: American Cancer Society; 2010.
-
- Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110(10):3540–3546. - PubMed
-
- Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22(6):1200–1206. - PubMed
-
- de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358–3363. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

