In silico analysis identifies a novel role for androgens in the regulation of human endometrial apoptosis
- PMID: 21865353
- PMCID: PMC3380091
- DOI: 10.1210/jc.2011-0272
In silico analysis identifies a novel role for androgens in the regulation of human endometrial apoptosis
Abstract
Context: The endometrium is a multicellular, steroid-responsive tissue that undergoes dynamic remodeling every menstrual cycle in preparation for implantation and, in absence of pregnancy, menstruation. Androgen receptors are present in the endometrium.
Objective: The objective of the study was to investigate the impact of androgens on human endometrial stromal cells (hESC).
Design: Bioinformatics was used to identify an androgen-regulated gene set and processes associated with their function. Regulation of target genes and impact of androgens on cell function were validated using primary hESC.
Setting: The study was conducted at the University Research Institute.
Patients: Endometrium was collected from women with regular menses; tissues were used for recovery of cells, total mRNA, or protein and for immunohistochemistry.
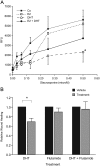
Results: A new endometrial androgen target gene set (n = 15) was identified. Bioinformatics revealed 12 of these genes interacted in one pathway and identified an association with control of cell survival. Dynamic androgen-dependent changes in expression of the gene set were detected in hESC with nine significantly down-regulated at 2 and/or 8 h. Treatment of hESC with dihydrotestosterone reduced staurosporine-induced apoptosis and cell migration/proliferation.
Conclusions: Rigorous in silico analysis resulted in identification of a group of androgen-regulated genes expressed in human endometrium. Pathway analysis and functional assays suggest androgen-dependent changes in gene expression may have a significant impact on stromal cell proliferation, migration, and survival. These data provide the platform for further studies on the role of circulatory or local androgens in the regulation of endometrial function and identify androgens as candidates in the pathogenesis of common endometrial disorders including polycystic ovarian syndrome, cancer, and endometriosis.
Figures



References
-
- Critchley HO, Saunders PT. 2009. Hormone receptor dynamics in a receptive human endometrium. Reprod Sci 16:191–199 - PubMed
-
- Burger HG. 2002. Androgen production in women. Fertil Steril 77(Suppl 4):S3–S5 - PubMed
-
- Abraham GE. 1974. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab 39:340–346 - PubMed
-
- Ito K, Suzuki T, Akahira J, Moriya T, Kaneko C, Utsunomiya H, Yaegashi N, Okamura K, Sasano H. 2002. Expression of androgen receptor and 5α-reductases in the human normal endometrium and its disorders. Int J Cancer 99:652–657 - PubMed
-
- Milne SA, Henderson TA, Kelly RW, Saunders PT, Baird DT, Critchley HO. 2005. Leukocyte populations and steroid receptor expression in human first-trimester decidua: regulation by antiprogestin and prostaglandin E analog. J Clin Endocrinol Metab 90:4315–4321 - PubMed

