Clinical review#: Lipodystrophies: genetic and acquired body fat disorders
- PMID: 21865368
- PMCID: PMC7673254
- DOI: 10.1210/jc.2011-1159
Clinical review#: Lipodystrophies: genetic and acquired body fat disorders
Abstract
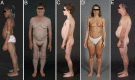
Context: Lipodystrophies are heterogeneous, genetic or acquired disorders characterized by selective loss of body fat and predisposition to insulin resistance. The extent of fat loss determines the severity of associated metabolic complications such as diabetes mellitus, hypertriglyceridemia, and hepatic steatosis.
Evidence acquisition and synthesis: Both original and review articles were found via PubMed search reporting on clinical features and management of various types of lipodystrophies and were integrated with the author's knowledge of the field.
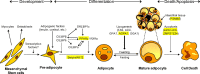
Conclusion: The autosomal recessive congenital generalized lipodystrophy and autosomal dominant familial partial lipodystrophy (FPL) are the two most common types of genetic lipodystrophies. Mutations in AGPAT2, BSCL2, CAV1, and PTRF have been reported in congenital generalized lipodystrophy and in LMNA, PPARG, AKT2, and PLIN1 in FPL. CIDEC is the disease gene for autosomal recessive, FPL and LMNA and ZMPSTE24 for autosomal recessive, mandibuloacral dysplasia-associated lipodystrophy. Recently, an autosomal recessive autoinflammatory lipodystrophy syndrome was reported to be due to PSMB8 mutation. Molecular genetic bases of many rare forms of genetic lipodystrophies remain to be elucidated. The most prevalent subtype of acquired lipodystrophy currently occurs with prolonged duration of protease inhibitor-containing, highly-active antiretroviral therapy in HIV-infected patients. The acquired generalized and partial lipodystrophies are mainly autoimmune in origin and display complement abnormalities. Localized lipodystrophies occur due to drug or vaccine injections, pressure, panniculitis, and other unknown reasons. The current management includes cosmetic surgery and early identification and treatment of metabolic and other complications with diet, exercise, hypoglycemic drugs, and lipid-lowering agents.
Figures

References
-
- Garg A. 2000. Lipodystrophies. Am J Med 108:143–152 - PubMed
-
- Garg A. 2004. Acquired and inherited lipodystrophies. N Engl J Med 350:1220–1234 - PubMed
-
- Mitchell SW. 1885. Singular case of absence of adipose matter in the upper half of the body. Am J Med Sci 90:105–106
-
- Ziegler LH. 1928. Lipodystrophies: report of seven cases. Brain 51:145–167
-
- Berardinelli W. 1954. An undiagnosed endocrinometabolic syndrome: report of 2 cases. J Clin Endocrinol Metab 14:193–204 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

