Rapid multiplexed immunoassay for simultaneous serodiagnosis of HIV-1 and coinfections
- PMID: 21865431
- PMCID: PMC3187301
- DOI: 10.1128/JCM.00970-11
Rapid multiplexed immunoassay for simultaneous serodiagnosis of HIV-1 and coinfections
Abstract
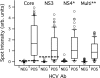
Diagnosis of opportunistic infections in HIV-infected individuals remains a major public health challenge, particularly in resource-limited settings. Here, we describe a rapid diagnostic system that delivers a panel of serologic immunoassay results using a single drop of blood, serum, or plasma. The system consists of disposable cartridges and a simple reader instrument, based on an innovative implementation of planar waveguide imaging technology. The cartridge incorporates a microarray of recombinant antigens and antibody controls in a fluidic channel, providing multiple parallel fluorescence immunoassay results for a single sample. This study demonstrates system performance by delivering antibody (Ab) reactivity results simultaneously for multiple antigens of HIV-1, Treponema pallidum (syphilis), and hepatitis C virus (HCV) in a collection of clinical serum, plasma, and whole-blood samples. By plotting antibody reactivity (fluorescence intensity) for known positive and negative samples, empirical reactivity cutoff values were defined. The HIV-1 assay shows 100% agreement with known seroreactivity for a collection of 82 HIV Ab-positive and 142 HIV Ab-negative samples, including multiple samples with HCV and syphilis coinfection. The treponema-specific syphilis assay correctly identifies 67 of 68 T. pallidum Ab-positive and 100 of 102 T. pallidum Ab-negative samples, and the HCV assay correctly identifies 59 of 60 HCV Ab-positive and 120 of 121 HCV Ab-negative samples. Multiplexed assay performance for whole-blood samples is also demonstrated. The ability to diagnose HIV and opportunistic infections simultaneously at the point of care should lead to more effective therapy decisions and improved linkage to care.
Figures




References
-
- Aach R. D., et al. 1991. Hepatitis C virus infection in post-transfusion hepatitis: an analysis with first- and second-generation assays. N. Engl. J. Med. 325:1325–1329 - PubMed
-
- Allain J.-P. 1998. The status of hepatitis C virus screening. Transfus. Med. Rev. 12:46–55 - PubMed
-
- Anderson D. A., Crowe S. M., Garcia M. 2011. Point-of-care testing. Curr. HIV/AIDS Rep. 8:31–37 - PubMed
-
- Chamot E., et al. 1990. Loss of antibodies against hepatitis C virus in HIV-seropositive intravenous drug users. AIDS 4:1275–1277 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

