Orientation selectivity of synaptic input to neurons in mouse and cat primary visual cortex
- PMID: 21865476
- PMCID: PMC3202243
- DOI: 10.1523/JNEUROSCI.2039-11.2011
Orientation selectivity of synaptic input to neurons in mouse and cat primary visual cortex
Erratum in
- J Neurosci. 2011 Oct 12;31(41):14832
Abstract
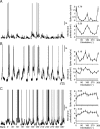
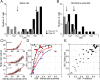
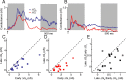
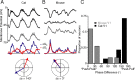
Primary visual cortex (V1) is the site at which orientation selectivity emerges in mammals: visual thalamus afferents to V1 respond equally to all stimulus orientations, whereas their target V1 neurons respond selectively to stimulus orientation. The emergence of orientation selectivity in V1 has long served as a model for investigating cortical computation. Recent evidence for orientation selectivity in mouse V1 opens cortical computation to dissection by genetic and imaging tools, but also raises two essential questions: (1) How does orientation selectivity in mouse V1 neurons compare with that in previously described species? (2) What is the synaptic basis for orientation selectivity in mouse V1? A comparison of orientation selectivity in mouse and in cat, where such measures have traditionally been made, reveals that orientation selectivity in mouse V1 is weaker than in cat V1, but that spike threshold plays a similar role in narrowing selectivity between membrane potential and spike rate. To uncover the synaptic basis for orientation selectivity, we made whole-cell recordings in vivo from mouse V1 neurons, comparing neuronal input selectivity-based on membrane potential, synaptic excitation, and synaptic inhibition-to output selectivity based on spiking. We found that a neuron's excitatory and inhibitory inputs are selective for the same stimulus orientations as is its membrane potential response, and that inhibitory selectivity is not broader than excitatory selectivity. Inhibition has different dynamics than excitation, adapting more rapidly. In neurons with temporally modulated responses, the timing of excitation and inhibition was different in mice and cats.
Figures



References
-
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84:909–926. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
