Antisense suppression of the small chloroplast protein CP12 in tobacco alters carbon partitioning and severely restricts growth
- PMID: 21865489
- PMCID: PMC3192581
- DOI: 10.1104/pp.111.183806
Antisense suppression of the small chloroplast protein CP12 in tobacco alters carbon partitioning and severely restricts growth
Abstract
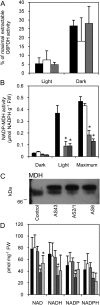
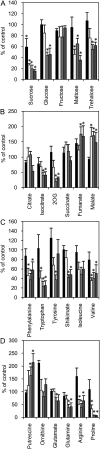
The thioredoxin-regulated chloroplast protein CP12 forms a multienzyme complex with the Calvin-Benson cycle enzymes phosphoribulokinase (PRK) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). PRK and GAPDH are inactivated when present in this complex, a process shown in vitro to be dependent upon oxidized CP12. The importance of CP12 in vivo in higher plants, however, has not been investigated. Here, antisense suppression of CP12 in tobacco (Nicotiana tabacum) was observed to impact on NAD-induced PRK and GAPDH complex formation but had little effect on enzyme activity. Additionally, only minor changes in photosynthetic carbon fixation were observed. Despite this, antisense plants displayed changes in growth rates and morphology, including dwarfism and reduced apical dominance. The hypothesis that CP12 is essential to separate oxidative pentose phosphate pathway activity from Calvin-Benson cycle activity, as proposed in cyanobacteria, was tested. No evidence was found to support this role in tobacco. Evidence was seen, however, for a restriction to malate valve capacity, with decreases in NADP-malate dehydrogenase activity (but not protein levels) and pyridine nucleotide content. Antisense repression of CP12 also led to significant changes in carbon partitioning, with increased carbon allocation to the cell wall and the organic acids malate and fumarate and decreased allocation to starch and soluble carbohydrates. Severe decreases were also seen in 2-oxoglutarate content, a key indicator of cellular carbon sufficiency. The data presented here indicate that in tobacco, CP12 has a role in redox-mediated regulation of carbon partitioning from the chloroplast and provides strong in vivo evidence that CP12 is required for normal growth and development in plants.
Figures





Similar articles
-
Antisense suppression of the small chloroplast protein CP12 in tobacco: a transcriptional viewpoint.Plant Signal Behav. 2011 Dec;6(12):2026-30. doi: 10.4161/psb.6.12.18055. Plant Signal Behav. 2011. PMID: 22112458 Free PMC article.
-
CP12-mediated protection of Calvin-Benson cycle enzymes from oxidative stress.Biochimie. 2014 Feb;97:228-37. doi: 10.1016/j.biochi.2013.10.018. Epub 2013 Nov 5. Biochimie. 2014. PMID: 24211189
-
Structural basis of light-induced redox regulation in the Calvin-Benson cycle in cyanobacteria.Proc Natl Acad Sci U S A. 2019 Oct 15;116(42):20984-20990. doi: 10.1073/pnas.1906722116. Epub 2019 Sep 30. Proc Natl Acad Sci U S A. 2019. PMID: 31570616 Free PMC article.
-
Thioredoxin-dependent regulation of photosynthetic glyceraldehyde-3-phosphate dehydrogenase: autonomous vs. CP12-dependent mechanisms.Photosynth Res. 2006 Sep;89(2-3):263-75. doi: 10.1007/s11120-006-9099-z. Epub 2006 Sep 22. Photosynth Res. 2006. PMID: 17031544 Review.
-
Calvin-Benson cycle regulation is getting complex.Trends Plant Sci. 2021 Sep;26(9):898-912. doi: 10.1016/j.tplants.2021.03.008. Epub 2021 Apr 20. Trends Plant Sci. 2021. PMID: 33893047 Review.
Cited by
-
A Trajectory of Discovery: Metabolic Regulation by the Conditionally Disordered Chloroplast Protein, CP12.Biomolecules. 2022 Jul 28;12(8):1047. doi: 10.3390/biom12081047. Biomolecules. 2022. PMID: 36008940 Free PMC article. Review.
-
The circadian oscillator in Synechococcus elongatus controls metabolite partitioning during diurnal growth.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):E1916-25. doi: 10.1073/pnas.1504576112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825710 Free PMC article.
-
Antisense suppression of the small chloroplast protein CP12 in tobacco: a transcriptional viewpoint.Plant Signal Behav. 2011 Dec;6(12):2026-30. doi: 10.4161/psb.6.12.18055. Plant Signal Behav. 2011. PMID: 22112458 Free PMC article.
-
In high-light-acclimated coffee plants the metabolic machinery is adjusted to avoid oxidative stress rather than to benefit from extra light enhancement in photosynthetic yield.PLoS One. 2014 Apr 14;9(4):e94862. doi: 10.1371/journal.pone.0094862. eCollection 2014. PLoS One. 2014. PMID: 24733284 Free PMC article.
-
Plastid thioredoxins: a "one-for-all" redox-signaling system in plants.Front Plant Sci. 2013 Nov 21;4:463. doi: 10.3389/fpls.2013.00463. Front Plant Sci. 2013. PMID: 24319449 Free PMC article. Review.
References
-
- Arnon DI, Hoagland DR. (1940) Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci 50: 463–485
-
- Avilan L, Gontero B, Lebreton S, Ricard J. (1997) Memory and imprinting effects in multienzyme complexes. I. Isolation, dissociation, and reassociation of a phosphoribulokinase-glyceraldehyde-3-phosphate dehydrogenase complex from Chlamydomonas reinhardtii chloroplasts. Eur J Biochem 246: 78–84 - PubMed
-
- Backhausen JE, Emmerlich A, Holtgrefe S, Horton P, Nast G, Rogers JJM, Muller-Rober B, Scheibe R. (1998) Transgenic potato plants with altered expression levels of chloroplast NADP-malate dehydrogenase: interactions between photosynthetic electron transport and malate metabolism in leaves and in isolated intact chloroplasts. Planta 207: 105–114
-
- Backhausen JE, Kitzmann C, Scheibe R. (1994) Competition between electron acceptors in photosynthesis: regulation of the malate valve during CO2 fixation and nitrite reduction. Photosynth Res 42: 75–86 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

