Potent kinetic stabilizers that prevent transthyretin-mediated cardiomyocyte proteotoxicity
- PMID: 21865539
- PMCID: PMC3227540
- DOI: 10.1126/scitranslmed.3002473
Potent kinetic stabilizers that prevent transthyretin-mediated cardiomyocyte proteotoxicity
Abstract
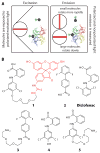
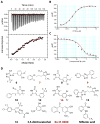
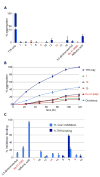
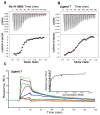
A valine-to-isoleucine mutation at position 122 of the serum protein transthyretin (TTR), found in 3 to 4% of African Americans, alters its stability, leading to amyloidogenesis and cardiomyopathy. In addition, 10 to 15% of individuals older than 65 years develop senile systemic amyloidosis and cardiac TTR deposits because of wild-type TTR amyloidogenesis. Although several drugs are in development, no approved therapies for TTR amyloid cardiomyopathy are yet available, so the identification of additional compounds that prevent amyloid-mediated cardiotoxicity is needed. To this aim, we developed a fluorescence polarization-based high-throughput screen and used it to identify several new chemical scaffolds that target TTR. These compounds were potent kinetic stabilizers of TTR and prevented TTR tetramer dissociation, partial unfolding, and aggregation of both wild type and the most common cardiomyopathy-associated TTR mutant, V122I-TTR. High-resolution co-crystal structures and characterization of the binding energetics revealed how these diverse structures bound to tetrameric TTR. These compounds effectively inhibited the proteotoxicity of V122I-TTR toward human cardiomyocytes. Several of these ligands stabilized TTR in human serum more effectively than diflunisal, which is a well-studied inhibitor of TTR aggregation, and may be promising leads for the treatment or prevention of TTR-mediated cardiomyopathy.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures

Comment in
-
Lead identification: Keeping tetramers together.Nat Rev Drug Discov. 2011 Oct 14;10(11):816. doi: 10.1038/nrd3586. Nat Rev Drug Discov. 2011. PMID: 21997752 No abstract available.
References
-
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. - PubMed
-
- Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. - PubMed
-
- Johnson SM, et al. Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses. Acc Chem Res. 2005;38:911–921. - PubMed
-
- Monaco HL, Rizzi M, Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. 1995;268:1039–1041. - PubMed
-
- Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

