Age-dependent susceptibility of chromosome cohesion to premature separase activation in mouse oocytes
- PMID: 21865557
- PMCID: PMC3223255
- DOI: 10.1095/biolreprod.111.094094
Age-dependent susceptibility of chromosome cohesion to premature separase activation in mouse oocytes
Abstract
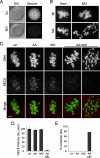
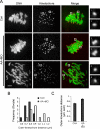
A hypothesis to explain the maternal age-dependent increase in formation of aneuploid eggs is deterioration of chromosome cohesion. Although several lines of evidence are consistent with this hypothesis, whether cohesion is actually reduced in naturally aged oocytes has not been directly tested by any experimental perturbation. To directly target cohesion, we increased the activity of separase, the protease that cleaves the meiotic cohesin REC8, in oocytes. We show that cohesion is more susceptible to premature separase activation in old oocytes than in young oocytes, demonstrating that cohesion is significantly reduced. Furthermore, cohesion is protected by two independent mechanisms that inhibit separase, securin and an inhibitory phosphorylation of separase by CDK1; both mechanisms must be disrupted to prematurely activate separase. With the continual loss of cohesins from chromosomes that occurs throughout the natural reproductive lifespan, tight regulation of separase in oocytes may be particularly important to maintain cohesion and prevent aneuploidy.
Figures
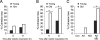
References
-
- Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet 2007; 16(spec no. 2): R203 R208 - PubMed
-
- Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet 2005; 37: 1351 1355 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

