Longitudinal assessment of oxaliplatin-induced neuropathy
- PMID: 21865571
- PMCID: PMC3171958
- DOI: 10.1212/WNL.0b013e31822cfc59
Longitudinal assessment of oxaliplatin-induced neuropathy
Abstract
Objectives: To characterize the natural history of oxaliplatin-associated neuropathy (ON) and determine whether intraepidermal nerve fiber density (IENFD) is a sensitive measure of neuropathy progression. In addition, we sought to assess the potential of ON as a neuroprotection model and gain insight into the relationship between axon loss and neuropathic symptoms.
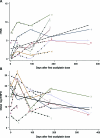
Methods: Eight subjects receiving oxaliplatin for advanced colorectal cancer were prospectively followed prior to starting chemotherapy and at 30, 90, 180, and 360 days (180 days after completing treatment). Electrophysiology, punch biopsies, symptom assessment, and examinations with calculation of a reduced total neuropathy score (rTNS) were performed at each time point. Changes over time were assessed through Poisson regression for IENFD and a mixed effects model for rTNS and electrophysiology measures.
Results: The distal leg IENFD, rTNS, peroneal, and sural amplitudes were all significantly reduced over time, while conduction velocity (peroneal and sural) and distal thigh IENFD were not. Measures of axon loss continued to worsen following discontinuation of oxaliplatin. Five of 8 subjects reported prominent symptoms associated with oxaliplatin administration.
Conclusions: This study demonstrates that oxaliplatin is associated with mild, sensory, and motor axon loss that may not be reversible. Axonal loss was detected by electrophysiology, rTNS, and distal leg IENFD. Several subjects reported prominent sensory symptoms that were not associated with axon loss, and that may or may not represent neuropathy. ON is an attractive paradigm for neuroprotection studies and the distal leg IENFD is an objective measure that requires minimal subject participation or study site expertise.
Figures

Comment in
-
Longitudinal assessment of oxaliplatin-induced neuropathy.Neurology. 2012 Jan 10;78(2):152. doi: 10.1212/01.wnl.0000410913.88642.bf. Neurology. 2012. PMID: 22232054 No abstract available.
References
-
- Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann Pharmacother 2005;39:128–135 - PubMed
-
- Gamelin E, Gamelin L, Bossi L, Quasthoff S. Clinical aspects and molecular basis of oxaliplatin neurotoxicity: current management and development of preventive measures. Semin Oncol 2002;29:21–33 - PubMed
-
- Diaz-Rubio E, Sastre J, Zaniboni A, et al. Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: a phase II multicentric study. Ann Oncol 1998;9:105–108 - PubMed
-
- Machover D, Diaz-Rubio E, de Gramont A, et al. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol 1996;7:95–98 - PubMed
-
- Becouarn Y, Ychou M, Ducreux M, et al. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients: Digestive Group of French Federation of Cancer Centers. J Clin Oncol 1998;16:2739–2744 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
