Hypoxic culture and in vivo inflammatory environments affect the assumption of pericyte characteristics by human adipose and bone marrow progenitor cells
- PMID: 21865587
- PMCID: PMC3233793
- DOI: 10.1152/ajpcell.00460.2010
Hypoxic culture and in vivo inflammatory environments affect the assumption of pericyte characteristics by human adipose and bone marrow progenitor cells
Abstract
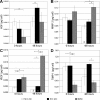
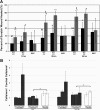
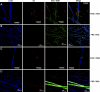
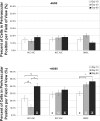
Previous studies have shown that exposure to a hypoxic in vitro environment increases the secretion of pro-angiogenic growth factors by human adipose-derived stromal cells (hASCs) [Cao Y, et al., Biochem Biophys Res Commun 332: 370-379, 2005; Kokai LE, et al., Plast Reconstr Surg 116: 1453-1460, 2005; Park BS, et al., Biomed Res (Tokyo) 31: 27-34, 2010; Rasmussen JG, et al., Cytotherapy 13: 318-328, 2010; Rehman J, et al., Circulation 109: 1292-1298, 2004]. Previously, it has been demonstrated that hASCs can differentiate into pericytes and promote microvascular stability and maintenance during angiogenesis in vivo (Amos PJ, et al., Stem Cells 26: 2682-2690, 2008; Traktuev DO, et al., Circ Res 102: 77-85, 2008). In this study, we tested the hypotheses that angiogenic induction can be increased and pericyte differentiation decreased by pretreatment of hASCs with hypoxic culture and that hASCs are similar to human bone marrow-derived stromal cells (hBMSCs) in these regards. Our data confirms previous studies showing that hASCs: 1) secrete pro-angiogenic proteins, which are upregulated following culture in hypoxia, and 2) migrate up gradients of PDGF-BB in vitro, while showing for the first time that a rat mesenteric model of angiogenesis induced by 48/80 increases the propensity of both hASCs and hBMSCs to assume perivascular phenotypes following injection. Moreover, culture of both cell types in hypoxia before injection results in a biphasic vascular length density response in this model of inflammation-induced angiogenesis. The effects of hypoxia and inflammation on the phenotype of adult progenitor cells impacts both the therapeutic and the basic science applications of the cell types, as hypoxia and inflammation are common features of natural and pathological vascular compartments in vivo.
Figures

References
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 97: 512–523, 2005 - PubMed
-
- Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332: 370–379, 2005 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

