Mia40-dependent oxidation of cysteines in domain I of Ccs1 controls its distribution between mitochondria and the cytosol
- PMID: 21865594
- PMCID: PMC3192855
- DOI: 10.1091/mbc.E11-04-0293
Mia40-dependent oxidation of cysteines in domain I of Ccs1 controls its distribution between mitochondria and the cytosol
Abstract
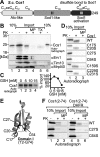
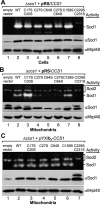
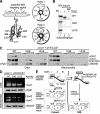
Superoxide dismutase 1 (Sod1) is an important antioxidative enzyme that converts superoxide anions to hydrogen peroxide and water. Active Sod1 is a homodimer containing one zinc ion, one copper ion, and one disulfide bond per subunit. Maturation of Sod1 depends on its copper chaperone (Ccs1). Sod1 and Ccs1 are dually localized proteins that reside in the cytosol and in the intermembrane space of mitochondria. The import of Ccs1 into mitochondria depends on the mitochondrial disulfide relay system. However, the exact mechanism of this import process has been unclear. In this study we detail the import and folding pathway of Ccs1 and characterize its interaction with the oxidoreductase of the mitochondrial disulfide relay Mia40. We identify cysteines at positions 27 and 64 in domain I of Ccs1 as critical for mitochondrial import and interaction with Mia40. On interaction with Mia40, these cysteines form a structural disulfide bond that stabilizes the overall fold of domain I. Although the cysteines are essential for the accumulation of functional Ccs1 in mitochondria, they are dispensable for the enzymatic activity of cytosolic Ccs1. We propose a model in which the Mia40-mediated oxidative folding of domain I controls the cellular distribution of Ccs1 and, consequently, active Sod1.
Figures



References
-
- Appenzeller-Herzog C, Ellgaard L. In vivo reduction-oxidation state of protein disulfide isomerase: the two active sites independently occur in the reduced and oxidized forms. Antioxid Redox Signal. 2008;10:55–64. - PubMed
-
- Bien M, Longen S, Wagener N, Chwalla I, Herrmann JM, Riemer J. Mitochondrial disulfide bond formation is driven by intersubunit electron transfer in Erv1 and proofread by glutathione. Mol Cell. 2010;37:516–528. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

