ARHGAP18, a GTPase-activating protein for RhoA, controls cell shape, spreading, and motility
- PMID: 21865595
- PMCID: PMC3192863
- DOI: 10.1091/mbc.E11-04-0364
ARHGAP18, a GTPase-activating protein for RhoA, controls cell shape, spreading, and motility
Abstract
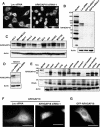
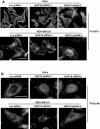
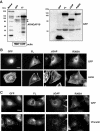
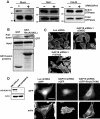
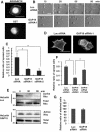
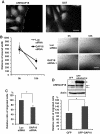
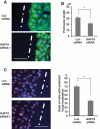
Rho GTPases are molecular switches that transmit biochemical signals in response to extracellular stimuli to elicit changes in the actin cytoskeleton. Rho GTPases cycle between an active, GTP-bound state and an inactive, GDP-bound state. These states are regulated by two distinct families of proteins-guanine nucleotide exchange factors and GTPase-activating proteins (GAPs). We studied the role of a previously uncharacterized GAP, ARHGAP18 (MacGAP). Overexpression of ARHGAP18 suppressed the activity of RhoA and disrupted stress fiber formation. Conversely, silencing of ARHGAP18 by small interfering RNA transfection-enhanced stress fiber formation and induced rounding of cells. We examined the role of ARHGAP18 in cell spreading and migration. Immunofluorescence analysis revealed that ARHGAP18 was localized to the leading edge during cell spreading and migration. ARHGAP18-knockdown cells showed impaired spreading, premature formation of stress fibers, and sustained activation of RhoA upon cell attachment. In addition, knockdown and overexpression of ARHGAP18 resulted in the inhibition and promotion of cell migration, respectively. Furthermore, ARHGAP18 was required for the polarization of cells for migration. Our results define ARHGAP18 as one of the crucial factors for the regulation of RhoA for the control of cell shape, spreading, and migration.
Figures
Similar articles
-
ARHGAP18 Downregulation by miR-200b Suppresses Metastasis of Triple-Negative Breast Cancer by Enhancing Activation of RhoA.Cancer Res. 2017 Aug 1;77(15):4051-4064. doi: 10.1158/0008-5472.CAN-16-3141. Epub 2017 Jun 15. Cancer Res. 2017. PMID: 28619708
-
Advanced glycosylation end products (AGEs) controls proliferation, invasion and permeability through orchestrating ARHGAP18/RhoA pathway in human umbilical vein endothelial cells.Glycoconj J. 2020 Apr;37(2):209-219. doi: 10.1007/s10719-020-09908-0. Epub 2020 Feb 3. Glycoconj J. 2020. PMID: 32016689
-
LARG GEF and ARHGAP18 orchestrate RhoA activity to control mesenchymal stem cell lineage.Bone. 2018 Feb;107:172-180. doi: 10.1016/j.bone.2017.12.001. Epub 2017 Dec 5. Bone. 2018. PMID: 29208526 Free PMC article.
-
FilGAP and its close relatives: a mediator of Rho-Rac antagonism that regulates cell morphology and migration.Biochem J. 2013 Jul 1;453(1):17-25. doi: 10.1042/BJ20130290. Biochem J. 2013. PMID: 23763313 Review.
-
[The Rho protein family and its role in the cellular cytoskeleton].Postepy Hig Med Dosw (Online). 2008 Mar 10;62:110-7. Postepy Hig Med Dosw (Online). 2008. PMID: 18334926 Review. Polish.
Cited by
-
Genomic and Reverse Translational Analysis Discloses a Role for Small GTPase RhoA Signaling in the Pathogenesis of Schizophrenia: Rho-Kinase as a Novel Drug Target.Int J Mol Sci. 2023 Oct 26;24(21):15623. doi: 10.3390/ijms242115623. Int J Mol Sci. 2023. PMID: 37958606 Free PMC article. Review.
-
A Screen for PKN3 Substrates Reveals an Activating Phosphorylation of ARHGAP18.Int J Mol Sci. 2020 Oct 20;21(20):7769. doi: 10.3390/ijms21207769. Int J Mol Sci. 2020. PMID: 33092266 Free PMC article.
-
ARHGAP18: an endogenous inhibitor of angiogenesis, limiting tip formation and stabilizing junctions.Small GTPases. 2014;5(3):1-15. doi: 10.4161/21541248.2014.975002. Small GTPases. 2014. PMID: 25425145 Free PMC article.
-
A parasite DNA binding protein with potential to influence disease susceptibility acts as an analogue of mammalian HMGA transcription factors.PLoS One. 2023 Jun 5;18(6):e0286526. doi: 10.1371/journal.pone.0286526. eCollection 2023. PLoS One. 2023. PMID: 37276213 Free PMC article.
-
Mechanical suppression of breast cancer cell invasion and paracrine signaling to osteoclasts requires nucleo-cytoskeletal connectivity.Bone Res. 2020 Nov 17;8(1):40. doi: 10.1038/s41413-020-00111-3. Bone Res. 2020. PMID: 33298883 Free PMC article.
References
-
- Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res. 2002;35:239–246. - PubMed
-
- Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10:719–722. - PubMed
-
- Barrett T, Xiao B, Dodson EJ, Dodson G, Ludbrook SB, Nurmahomed K, Gamblin SJ, Musacchio A, Smerdon SJ, Eccleston JF. The structure of the GTPase-activating domain from p50rhoGAP. Nature. 1997;385:458–461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

