The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation
- PMID: 21865599
- PMCID: PMC3192859
- DOI: 10.1091/mbc.E11-05-0393
The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation
Abstract
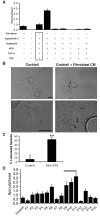
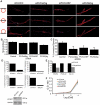
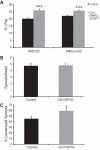
A role for fibroblasts in physiological and pathological angiogenesis is now well recognized; however, the precise mechanisms underlying their action have not been determined. Using an in vitro angiogenesis model in combination with a candidate gene approach, column chromatography, and mass spectrometry, we identify two classes of fibroblast-derived factors--one that supports vessel sprouting but not lumen formation, and one that promotes lumen formation. In the absence of fibroblasts a combination of angiopoietin-1, angiogenin, hepatocyte growth factor, transforming growth factor-α, and tumor necrosis factor drives robust endothelial cell (EC) sprouting; however, lumens fail to form. Subsequent addition of fibroblast-conditioned medium restores lumenogenesis. Using small interfering RNA-mediated knockdown, we show that five genes expressed in fibroblasts--collagen I, procollagen C endopeptidase enhancer 1, secreted protein acidic and rich in cysteine, transforming growth factor-β-induced protein ig-h3, and insulin growth factor-binding protein 7--are necessary for lumen formation. Moreover, lumen formation can be rescued by addition of purified protein to knockdown cultures. Finally, using rheology, we demonstrate that the presence of these matricellular proteins results in significantly stiffer gels, which correlates with enhanced lumen formation. These findings highlight the critical role that fibroblast-derived extracellular matrix components play in EC lumen formation and provide potential insight into the role of fibroblasts in the tumor microenvironment.
Figures


References
-
- Aitkenhead M, Wang SJ, Nakatsu MN, Mestas J, Heard C, Hughes CC. Identification of endothelial cell genes expressed in an in vitro model of angiogenesis: induction of ESM-1, (beta)ig-h3, and NrCAM. Microvasc Res. 2002;63:159–171. - PubMed
-
- Albini A, Magnani E, Noonan DM. The tumor microenvironment: biology of a complex cellular and tissue society. Q J Nucl Med Mol Imaging. 2010;54:244–248. - PubMed
-
- Berthod F, Germain L, Tremblay N, Auger FA. Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J Cell Physiol. 2006;207:491–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

