A uroguanylin-GUCY2C endocrine axis regulates feeding in mice
- PMID: 21865642
- PMCID: PMC3223926
- DOI: 10.1172/JCI57925
A uroguanylin-GUCY2C endocrine axis regulates feeding in mice
Abstract
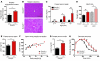
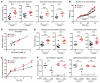
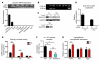
Intestinal enteroendocrine cells are critical to central regulation of caloric consumption, since they activate hypothalamic circuits that decrease appetite and thereby restrict meal size by secreting hormones in response to nutrients in the gut. Although guanylyl cyclase and downstream cGMP are essential regulators of centrally regulated feeding behavior in invertebrates, the role of this primordial signaling mechanism in mammalian appetite regulation has eluded definition. In intestinal epithelial cells, guanylyl cyclase 2C (GUCY2C) is a transmembrane receptor that makes cGMP in response to the paracrine hormones guanylin and uroguanylin, which regulate epithelial cell dynamics along the crypt-villus axis. Here, we show that silencing of GUCY2C in mice disrupts satiation, resulting in hyperphagia and subsequent obesity and metabolic syndrome. This defined an appetite-regulating uroguanylin-GUCY2C endocrine axis, which we confirmed by showing that nutrient intake induces intestinal prouroguanylin secretion into the circulation. The prohormone signal is selectively decoded in the hypothalamus by proteolytic liberation of uroguanylin, inducing GUCY2C signaling and consequent activation of downstream anorexigenic pathways. Thus, evolutionary diversification of primitive guanylyl cyclase signaling pathways allows GUCY2C to coordinate endocrine regulation of central food acquisition pathways with paracrine control of intestinal homeostasis. Moreover, the uroguanylin-GUCY2C endocrine axis may provide a therapeutic target to control appetite, obesity, and metabolic syndrome.
Figures




Comment in
-
Uroguanylin: how the gut got another satiety hormone.J Clin Invest. 2011 Sep;121(9):3384-6. doi: 10.1172/JCI58297. Epub 2011 Aug 25. J Clin Invest. 2011. PMID: 21865649 Free PMC article.
References
-
- Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307(5717):1909–1914. - PubMed
-
- Carrithers SL, Parkinson SJ, Goldstein S, Park P, Robertson DC, Waldman SA. Escherichia coli heat-stable toxin receptors in human colonic tumors. Gastroenterology. 1994;107(6):1653–1661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

