Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut
- PMID: 21865643
- PMCID: PMC3163971
- DOI: 10.1172/JCI58186
Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut
Abstract
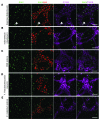
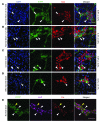
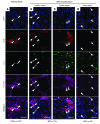
It is unclear whether neurogenesis occurs in the adult mammalian enteric nervous system (ENS). Neural crest-derived cells capable of forming multilineage colonies in culture, and neurons and glia upon transplantation into chick embryos, persist throughout adult life in the mammalian ENS. In this study we sought to determine the physiological function of these cells. We discovered that these cells could be identified based on CD49b expression and that they had characteristics of enteric glia, including p75, GFAP, S100B, and SOX10 expression. To test whether new neurons or glia arise in the adult gut under physiological conditions, we marked dividing progenitors with a thymidine analog in rodents under steady-state conditions, or during aging, pregnancy, dietary changes, hyperglycemia, or exercise. We also tested gut injuries including inflammation, irradiation, benzalkonium chloride treatment, partial gut stenosis, and glial ablation. We readily observed neurogenesis in a neurogenic region of the central nervous system, but not reproducibly in the adult ENS. Lineage tracing of glial cells with GFAP-Cre and GFAP-CreERT2 also detected little or no adult ENS neurogenesis. Neurogenesis in the adult gut is therefore very limited under the conditions we studied. In contrast, ENS gliogenesis was readily observed under steady-state conditions and after injury. Adult enteric glia thus have the potential to form neurons and glia in culture but are fated to form mainly glia under physiological conditions and after the injuries we studied.
Figures



Comment in
-
Behind an enteric neuron there may lie a glial cell.J Clin Invest. 2011 Sep;121(9):3386-9. doi: 10.1172/JCI59573. Epub 2011 Aug 25. J Clin Invest. 2011. PMID: 21865648 Free PMC article.
References
-
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11(1):173–189. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

