Mouse models for Down syndrome-associated developmental cognitive disabilities
- PMID: 21865664
- PMCID: PMC3254039
- DOI: 10.1159/000329422
Mouse models for Down syndrome-associated developmental cognitive disabilities
Abstract
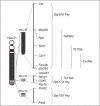
Down syndrome (DS) is mainly caused by the presence of an extra copy of human chromosome 21 (Hsa21) and is a leading genetic cause for developmental cognitive disabilities in humans. The mouse is a premier model organism for DS because the regions on Hsa21 are syntenically conserved with three regions in the mouse genome, which are located on mouse chromosome 10 (Mmu10), Mmu16 and Mmu17. With the advance of chromosomal manipulation technologies, new mouse mutants have been generated to mimic DS at both the genotypic and phenotypic levels. Further mouse-based molecular genetic studies in the future may lead to the unraveling of the mechanisms underlying DS-associated developmental cognitive disabilities, which would lay the groundwork for developing effective treatments for this phenotypic manifestation. In this review, we will discuss recent progress and future challenges in modeling DS-associated developmental cognitive disability in mice with an emphasis on hippocampus-related phenotypes.
Copyright © 2011 S. Karger AG, Basel.
Figures



References
-
- Ahn KJ, Jeong HK, Choi HS, Ryoo SR, Kim YJ, Goo JS, Choi SY, Han JS, Ha I, Song WJ. DYRK1A BAC transgenic mice show altered synaptic plasticity with learning and memory defects. Neurobiol Dis. 2006;22:463–472. - PubMed
-
- Akeson EC, Lambert JP, Narayanswami S, Gardiner K, Bechtel LJ, Davisson MT. Ts65Dn – localization of the translocation breakpoint and trisomic gene content in a mouse model for Down syndrome. Cytogenet Cell Genet. 2001;93:270–276. - PubMed
-
- Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, Bonnen PE, de Bakker PI, Deloukas P, Gabriel SB, Gwilliam R, Hunt S, Inouye M, Jia X, Palotie A, Parkin M, Whittaker P, Chang K, Hawes A, Lewis LR, Ren Y, Wheeler D, Muzny DM, Barnes C, Darvishi K, Hurles M, Korn JM, Kristiansson K, Lee C, McCarrol SA, Nemesh J, Keinan A, Montgomery SB, Pollack S, Price AL, Soranzo N, Gonzaga-Jauregui C, Anttila V, Brodeur W, Daly MJ, Leslie S, McVean G, Moutsianas L, Nguyen H, Zhang Q, Ghori MJ, McGinnis R, McLaren W, Takeuchi F, Grossman SR, Shlyakhter I, Hostetter EB, Sabeti PC, Adebamowo CA, Foster MW, Gordon DR, Licinio J, Manca MC, Marshall PA, Matsuda I, Ngare D, Wang VO, Reddy D, Rotimi CN, Royal CD, Sharp RR, Zeng C, Brooks LD, McEwen JE. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. - PMC - PubMed
-
- Andrle M, Fiedler W, Rett A, Ambros P, Schweizer D. A case of trisomy 22 in Pongo pygmaeus. Cytogenet Cell Genet. 1979;24:1–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

