Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip
- PMID: 21866153
- PMCID: PMC3812235
- DOI: 10.1038/nature10346
Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip
Abstract
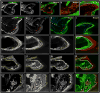
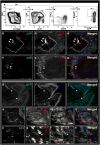
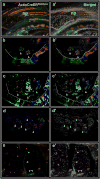
The regrowth of amputated limbs and the distal tips of digits represent models of tissue regeneration in amphibians, fish and mice. For decades it had been assumed that limb regeneration derived from the blastema, an undifferentiated pluripotent cell population thought to be derived from mature cells via dedifferentiation. Here we show that a wide range of tissue stem/progenitor cells contribute towards the restoration of the mouse distal digit. Genetic fate mapping and clonal analysis of individual cells revealed that these stem cells are lineage restricted, mimicking digit growth during development. Transplantation of cyan-fluorescent-protein-expressing haematopoietic stem cells, and parabiosis between genetically marked mice, confirmed that the stem/progenitor cells are tissue resident, including the cells involved in angiogenesis. These results, combined with those from appendage regeneration in other vertebrate subphyla, collectively demonstrate that tissue stem cells rather than pluripotent blastema cells are an evolutionarily conserved cellular mode for limb regeneration after amputation.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

