Celastrol inhibits aminoglycoside-induced ototoxicity via heat shock protein 32
- PMID: 21866174
- PMCID: PMC3181421
- DOI: 10.1038/cddis.2011.76
Celastrol inhibits aminoglycoside-induced ototoxicity via heat shock protein 32
Abstract
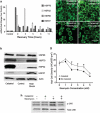
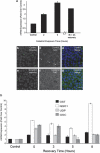
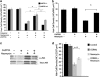
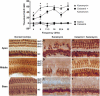
Hearing loss is often caused by death of the mechanosensory hair cells of the inner ear. Hair cells are susceptible to death caused by aging, noise trauma, and ototoxic drugs, including the aminoglycoside antibiotics and the antineoplastic agent cisplatin. Ototoxic drugs result in permanent hearing loss for over 500,000 Americans annually. We showed previously that induction of heat shock proteins (HSPs) inhibits both aminoglycoside- and cisplatin-induced hair cell death in whole-organ cultures of utricles from adult mice. In order to begin to translate these findings into a clinical therapy aimed at inhibiting ototoxic drug-induced hearing loss, we have now examined a pharmacological HSP inducer, celastrol. Celastrol induced upregulation of HSPs in utricles, and it provided significant protection against aminoglycoside-induced hair cell death in vitro and in vivo. Moreover, celastrol inhibited hearing loss in mice receiving systemic aminoglycoside treatment. Our data indicate that the major heat shock transcription factor HSF-1 is not required for celastrol-mediated protection. HSP32 (also called heme oxygenase-1, HO-1) is the primary mediator of the protective effect of celastrol. HSP32/HO-1 inhibits pro-apoptotic c-Jun N-terminal kinase (JNK) activation and hair cell death. Taken together, our data indicate that celastrol inhibits aminoglycoside ototoxicity via HSP32/HO-1 induction.
Figures

References
-
- Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13:119–126. - PubMed
-
- Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139:97–115. - PubMed
-
- Matsui JI, Gale JE, Warchol ME. Critical signaling events during the aminoglycoside-induced death of sensory hair cells in vitro. J Neurobiol. 2004;61:250–266. - PubMed
-
- Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

