Inhibition of Wnt signaling by cucurbitacin B in breast cancer cells: reduction of Wnt-associated proteins and reduced translocation of galectin-3-mediated β-catenin to the nucleus
- PMID: 21866566
- PMCID: PMC3245346
- DOI: 10.1002/jcb.23326
Inhibition of Wnt signaling by cucurbitacin B in breast cancer cells: reduction of Wnt-associated proteins and reduced translocation of galectin-3-mediated β-catenin to the nucleus
Abstract
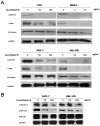
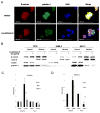
The cucurbitacins are tetracyclic triterpenes found in plants of the family Cucurbitaceae. Cucurbitacins have been shown to have anti-cancer and anti-inflamatory activities. We investigated the anti-cancer activity of cucurbitacin B extracted from Thai medicinal plant Trichosanthes cucumerina Linn. Cell viability was assessed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Results indicated that cucurbitacin B from T. cucumerina Linn. has a cytotoxic effect on breast cancer cell lines SKBR-3 and MCF-7 with an IC50 of 4.60 and 88.75 µg/ml, respectively. Growth inhibition was attributed to G2/M phase arrest and apoptosis. Cyclin D1, c-Myc, and β-catenin expression levels were reduced. Western blot analysis showed increased PARP cleavage and decreased Wnt-associated signaling molecules β-catenin, galectin-3, cyclin D1 and c-Myc, and corresponding changes in phosphorylated GSK-3β levels. Cucurbitacin B treatment inhibited translocation to the nucleus of β-catenin and galectin-3. The depletion of β-catenin and galectin-3 in the nucleus was confirmed by cellular protein fractionation. T-cell factor (TCF)/lymphoid enhancer factor (LEF)-dependent transcriptional activity was disrupted in cucurbitacin B treated cells as tested by a TCF reporter assay. The relative luciferase activity was reduced when we treated cells with cucurbitacin B compound for 24 h. Our data suggest that cucurbitacin B may in part induce apoptosis and exert growth inhibitory effect via interruption the Wnt signaling.
Copyright © 2011 Wiley Periodicals, Inc.
Figures





References
-
- Arawwawala M, Thabrew I, Arambewela L, Handunnetti S. Anti-inflammatory activity of Trichosanthes cucumerina Linn. in rats. J Ethnopharmacol. 2010;131:538–543. - PubMed
-
- Blagosklonny MV, Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review) Int J Cancer. 1999;83:151–156. - PubMed
-
- Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99–166. - PubMed
-
- Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9:119–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

