Between-centre differences and treatment effects in randomized controlled trials: a case study in traumatic brain injury
- PMID: 21867540
- PMCID: PMC3170218
- DOI: 10.1186/1745-6215-12-201
Between-centre differences and treatment effects in randomized controlled trials: a case study in traumatic brain injury
Abstract
Background: In Traumatic Brain Injury (TBI), large between-centre differences in outcome exist and many clinicians believe that such differences influence estimation of the treatment effect in randomized controlled trial (RCTs). The aim of this study was to assess the influence of between-centre differences in outcome on the estimated treatment effect in a large RCT in TBI.
Methods: We used data from the MRC CRASH trial on the efficacy of corticosteroid infusion in patients with TBI. We analyzed the effect of the treatment on 14 day mortality with fixed effect logistic regression. Next we used random effects logistic regression with a random intercept to estimate the treatment effect taking into account between-centre differences in outcome. Between-centre differences in outcome were expressed with a 95% range of odds ratios (OR) for centres compared to the average, based on the variance of the random effects (tau2). A random effects logistic regression model with random slopes was used to allow the treatment effect to vary by centre. The variation in treatment effect between the centres was expressed in a 95% range of the estimated treatment ORs.
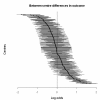
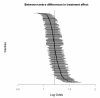
Results: In 9978 patients from 237 centres, 14-day mortality was 19.5%. Mortality was higher in the treatment group (OR = 1.22, p = 0.00010). Using a random effects model showed large between-centre differences in outcome (95% range of centre effects: 0.27- 3.71), but did not substantially change the estimated treatment effect (OR = 1.24, p = 0.00003). There was limited, although statistically significant, between-centre variation in the treatment effect (OR = 1.22, 95% treatment OR range: 1.17-1.26).
Conclusion: Large between-centre differences in outcome do not necessarily affect the estimated treatment effect in RCTs, in contrast to current beliefs in the clinical area of TBI.
Figures
Similar articles
-
Covariate adjustment increased power in randomized controlled trials: an example in traumatic brain injury.J Clin Epidemiol. 2012 May;65(5):474-81. doi: 10.1016/j.jclinepi.2011.08.012. Epub 2011 Dec 9. J Clin Epidemiol. 2012. PMID: 22169080 Free PMC article. Clinical Trial.
-
Validation of a prognostic score for early mortality in severe head injury cases.J Neurosurg. 2014 Dec;121(6):1314-22. doi: 10.3171/2014.7.JNS131874. Epub 2014 Sep 19. J Neurosurg. 2014. PMID: 25237737
-
Predicted Unfavorable Neurologic Outcome Is Overestimated by the Marshall Computed Tomography Score, Corticosteroid Randomization After Significant Head Injury (CRASH), and International Mission for Prognosis and Analysis of Clinical Trials in Traumatic Brain Injury (IMPACT) Models in Patients with Severe Traumatic Brain Injury Managed with Early Decompressive Craniectomy.World Neurosurg. 2017 May;101:554-558. doi: 10.1016/j.wneu.2017.02.051. Epub 2017 Feb 20. World Neurosurg. 2017. PMID: 28223249
-
Efficacy of progesterone for moderate to severe traumatic brain injury: a meta-analysis of randomized clinical trials.Sci Rep. 2015 Aug 25;5:13442. doi: 10.1038/srep13442. Sci Rep. 2015. PMID: 26304556 Free PMC article. Review.
-
Corticosteroids in acute traumatic brain injury: systematic review of randomised controlled trials.BMJ. 1997 Jun 28;314(7098):1855-9. doi: 10.1136/bmj.314.7098.1855. BMJ. 1997. PMID: 9224126 Free PMC article.
Cited by
-
Randomized Evaluation of Surgery in Elderly with Traumatic Acute SubDural Hematoma (RESET-ASDH trial): study protocol for a pragmatic randomized controlled trial with multicenter parallel group design.Trials. 2022 Mar 29;23(1):242. doi: 10.1186/s13063-022-06184-1. Trials. 2022. PMID: 35351178 Free PMC article.
-
Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program.J Neurotrauma. 2013 Oct 15;30(20):1737-46. doi: 10.1089/neu.2012.2802. Epub 2013 Jul 11. J Neurotrauma. 2013. PMID: 23731257 Free PMC article.
-
A nested mechanistic sub-study into the effect of tranexamic acid versus placebo on intracranial haemorrhage and cerebral ischaemia in isolated traumatic brain injury: study protocol for a randomised controlled trial (CRASH-3 Trial Intracranial Bleeding Mechanistic Sub-Study [CRASH-3 IBMS]).Trials. 2017 Jul 17;18(1):330. doi: 10.1186/s13063-017-2073-6. Trials. 2017. PMID: 28716153 Free PMC article. Clinical Trial.
-
Toward an international initiative for traumatic brain injury research.J Neurotrauma. 2013 Jul 15;30(14):1211-22. doi: 10.1089/neu.2013.2896. Epub 2013 Jul 11. J Neurotrauma. 2013. PMID: 23731282 Free PMC article.
-
Reference effect measures for quantifying, comparing and visualizing variation from random and fixed effects in non-normal multilevel models, with applications to site variation in medical procedure use and outcomes.BMC Med Res Methodol. 2018 Jul 6;18(1):74. doi: 10.1186/s12874-018-0517-7. BMC Med Res Methodol. 2018. PMID: 29980180 Free PMC article.
References
-
- Finkelstein EA, Corso PS, Miller TR. The incidence and economic burden of injuries in the United States. New York: Oxford University Press; 2006.
-
- Maas AI, Murray G, Henney H, Kassem N, Legrand V, Mangelus M, Muizelaar JP, Stocchetti N, Knoller N. Pharmos TBI investigators. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5(1):38–45. doi: 10.1016/S1474-4422(05)70253-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

